Althea’s Crystalomics® Technology enables the manufacture of highly concentrated, low volume formulations of protein-based therapeutics without affecting biological activity.
Althea determines the conditions for crystallization of your molecule, then refines and optimizes those conditions to allow scale-up and large scale GMP manufacturing of the final formulated product.
Althea’s Crystalomics® Technology is a portfolio of patents surrounding crystallization, cross-linking, and complexation of proteins for therapeutic use. It includes know-how for finding ideal crystallization conditions and stable crystalline formulations. It also includes technologies to develop GMP manufacturing processes for crystalline suspension drug products.
For more information, please send an email to: info@altheacmo.com
Screening for Crystallization Conditions
The FORMULATOR® - Screen Builder allows Althea’s Crystalomics® Team to rapidly make custom screens while the ROCK IMAGER 1000® - Crystallization Imager captures visible light and UV images on a scheduled basis. Althea’s proprietary technology and biophysical characterization capabilities facilitate smarter excipient screening and streamline the process of getting hits.
Scale Up and Production
Once microbatch conditions have been established at the 10 mL scale, crystallization is transferred to the EasyMax reactor, where Process Analytical Technology (PAT) via FBRM and PVM is used to monitor particle size distribution and capture images respectively, in real time. Temperature and stirring effects are also monitored for their impact on crystallization, up to a scale of 100 mL.
Larger vessels of 3 L and 5 L working volumes respectively.

Benefits of Crystalline Biologics
The traditional challenge of delivering soluble molecules at high concentrations, which can have adverse effects due to high viscosity, can be overcome by developing their crystallized counterparts. In addition, the biological activity of these crystallized proteins is indistinguishable from the soluble form. As a result, patients could potentially receive subcutaneous injections of the crystallized molecule much less frequently than they would for the intravenously administered soluble version.
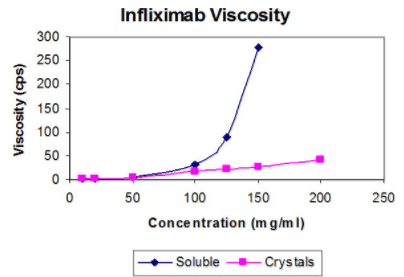
Figure 1 - Viscosity measurements of infliximab. The viscosity of soluble and crystalline suspensions of infliximab was measured by using the Cannon-Fenske viscometer according to the manufacturer’s instructions.
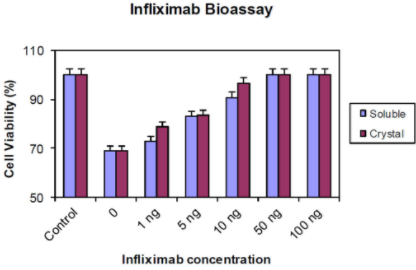
Figure 2 - In vitro bioactivity of infliximab. Cultured L-929 mouse fibroblast cells were detached, diluted to 2x105 cells per mL, and added to 96-well plates (100 μL per well). TNF-α neutralization assays were performed by incubating mouse fibroblast cells overnight in the presence of various concentrations of infliximab or dissolved crystals of infliximab. The number of viable cells was determined by using a commercially available cell proliferation assay kit.
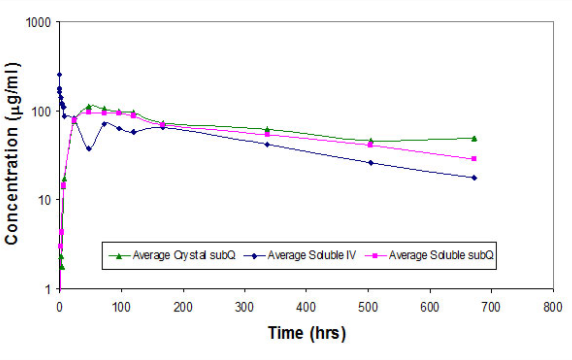
Figure 3 - Pharmacokinetic profile of infliximab administered in a soluble form either intravenous (IV) or subcutaneous (SC) or as a crystalline suspension SC. Approximately 100 μL of infliximab (20 mg/mL) was administered to BBDR_Wor rats having a body weight of 250 g to provide a dose of 8mg/kg. The maximum concentration (Cmax) for soluble infliximab was 257 μg/mL for the IV sample and 95 and 112 μg/mL for the SC soluble and crystalline samples, respectively. The half-life of soluble infliximab was 270 h for IV sample; the half-life of the SC soluble and crystalline infliximab was 390 and 779 h, respectively.