Nucleic acids, the fundamental molecules of life, store, transmit, and express genetic information. These biomolecules are indispensable tools in biotechnology and molecular biology. The manipulation of nucleic acids, such as in vitro RNA and DNA synthesis, underpins various applications, from basic research to clinical diagnostics, gene editing, biosynthesis, drug development, and therapeutics. The workflows for these diverse applications require concentration and diafiltration (or buffer exchange) as integral steps during reaction cleanup and final formulation. These steps significantly impact the quality, yield, and downstream applications of the final product.
In the in vitro mRNA synthesis workflow, concentration and diafiltration are essential for DNA template and mRNA purification. Diluted DNA templates are concentrated, and excess salts or impurities are removed through buffer exchange to optimize conditions for subsequent reactions. Similarly, the synthesized mRNA is formulated for downstream applications such as cell transfection or storage.
In vitro mRNA synthesis and purification
Likewise, even after purification, concentration and desalting are necessary for DNA processing workflows to formulate the DNA at optimal conditions, ensuring its structural integrity and effectiveness for intended applications. Given the importance of these processes, factors like concentration and buffer exchange efficacy, gentleness, time efficiency, scalability, and final yield need to be considered when choosing appropriate methods for concentration and diafiltration of nucleic acids.
Table below presents various techniques for diafiltration and final formulation of nucleic acid, with ultrafiltration emerging as the most suitable method. However, despite being a common method, ultrafiltration using dead-end devices (dead-end filtration or DEF) is limited by low filtration rates, scalability challenges, frequent sample loss, and manual interventions.
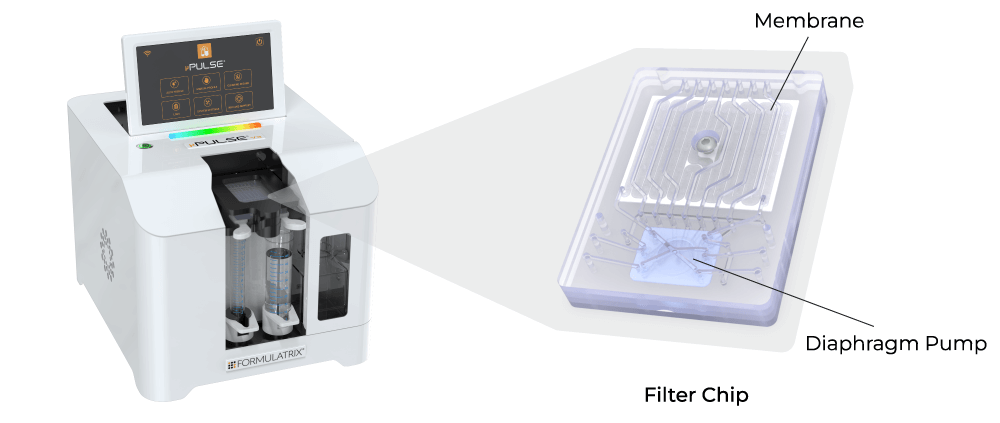
In contrast, ultrafiltration in the form of tangential flow filtration (TFF) offers higher filtration rates and scalability. However, traditional TFF systems are typically large and have high hold-up volumes, making them unsuitable for lab scale applications. To overcome these limitations, Formulatrix has developed the µPulse® - an automated and miniaturized TFF system designed specifically for sample concentration and buffer exchange at the lab scale.
| Considerations | Precipitation |
Column Chromatography | Evaporation | Lyophilization |
Ultrafiltration Dead-End TFF |
|---|---|---|---|---|---|
| Concentration Efficacy | |||||
| Diafiltration Efficacy | |||||
| Yield | |||||
| Gentleness | |||||
| Scalability | |||||
| Processing Time | |||||
| Cost Effectiveness |
Table: Methods for nucleic acid concentration and diafiltration
Nucleic Acid Processing Using the µPulse - TFF System
The Formulatrix µPulse is an automated and miniaturized TFF system explicitly designed for lab scale applications. It is well suited for processing RNA (siRNA, mRNA), cDNA, linear, and plasmid DNA. The entire fluid path is miniaturized on the filter chip by combining our patented microfluidic pumping technology with TFF. This has reduced the hold-up volume to just 0.65 mL, ensuring maximum hold-up recovery.
The customizable settings in µPulse, including adjustable pressures and pump parameters, provide a gentle yet highly efficient nucleic acid processing. The filter chips are available with modified polyethersulfone (mPES) and regenerated cellulose (RC) membranes that exhibit low fouling characteristics and are compatible with various sample types. Furthermore, the µPulse processes samples 4x faster than the dead-end centrifugal units and in a walk-away approach.
High-Throughput Nucleic Acid Processing with the aµtoPulse - TFF System
The aµtoPulse® is a fully automated, high-throughput TFF system with the world’s lowest hold-up volume of just 250 µL. It processes up to 54 samples per run, with up to 4 samples in parallel. Designed for flexibility, it handles starting volumes between 0.5 mL to 100 mL and can concentrate samples down to 250 µL with ±25 µL precision. The system supports up to four external buffer inputs or on-deck conical tubes, enabling automated multi-buffer diafiltration.
The advanced chip design with dual pumps delivers permeate flow rates up to 1.7x faster than the µPulse while minimizing shear. The chips are available with mPES (5–300 kDa) and RC (5–100 kDa) membranes, ensuring compatibility with a wide range of samples.
Each station offers independent regulation and monitoring of transmembrane pressure (0–32 psi), giving users full control over process gentleness and efficiency. The intuitive, browser-based software enables remote protocol setup, monitoring, and control, with secure data management compliant with 21 CFR Part 11 for GMP environments.
Publications
FAQs
TFF for plasmid DNA purification — is it efficient?
Yes. TFF is an efficient method for plasmid DNA purification, especially for concentration and buffer exchange. Gentle tangential flow and controlled transmembrane pressure retain large plasmid DNA while allowing salts and small impurities to pass through. Automated systems like the µPulse and the aµtoPulse offer low hold-up volumes, precise TMP control, and reproducible diafiltration, helping preserve plasmid integrity and the supercoiled structure while delivering high recovery. Compared to centrifugation or dialysis, TFF enables faster processing, higher yields, and scalable performance, making it well suited for research and process development workflows.
How efficient is TFF for plasmid DNA purification compared to precipitation?
Yes. TFF is an efficient and often superior alternative to precipitation for plasmid DNA purification. Unlike precipitation, which involves harsh chemical conditions, multiple manual steps, and variable recovery, TFF enables gentle, controlled concentration and buffer exchange using an appropriate MWCO membrane to retain plasmid DNA while removing salts, solvents, and impurities. Automated systems like the µPulse and the aµtoPulse provide precise TMP control, low hold-up volume, and reproducible processing, resulting in higher recovery, better plasmid integrity, and improved scalability.
What’s the recovery rate of circular DNA vectors using TFF?
Yes. TFF achieves high recovery rates for circular DNA vectors—typically above 95%—when optimized correctly. Recovery depends on membrane selection and process control. A 300–500 kDa MWCO ultrafiltration membrane made of low-binding modified PES or regenerated cellulose is recommended to retain plasmids or other circular DNA while removing impurities. Systems like the µPulse and aµtoPulse support gentle, automated processing to minimize shear stress and reduce DNA nicking or degradation. Key factors include sample viscosity, TMP, cross-flow velocity, and final concentration factor. Validate the process at small scale using agarose gel electrophoresis or HPLC before scaling up.