Vaccines are amongst the most powerful tools in modern medicine, designed to train the immune system to recognize and combat pathogens. From the eradication of smallpox to the global response against COVID-19, vaccines have consistently demonstrated their value in controlling infectious diseases. Their development, however, is a complex, multi-stage process involving careful design, rigorous testing, and robust manufacturing workflows to ensure safety, efficacy, and scalability. Sample concentration and buffer exchange are crucial steps in vaccine development, directly contributing to vaccine effectiveness. These processes increase product concentration, replace buffer systems to enhance stability and compatibility, and help remove residual contaminants.
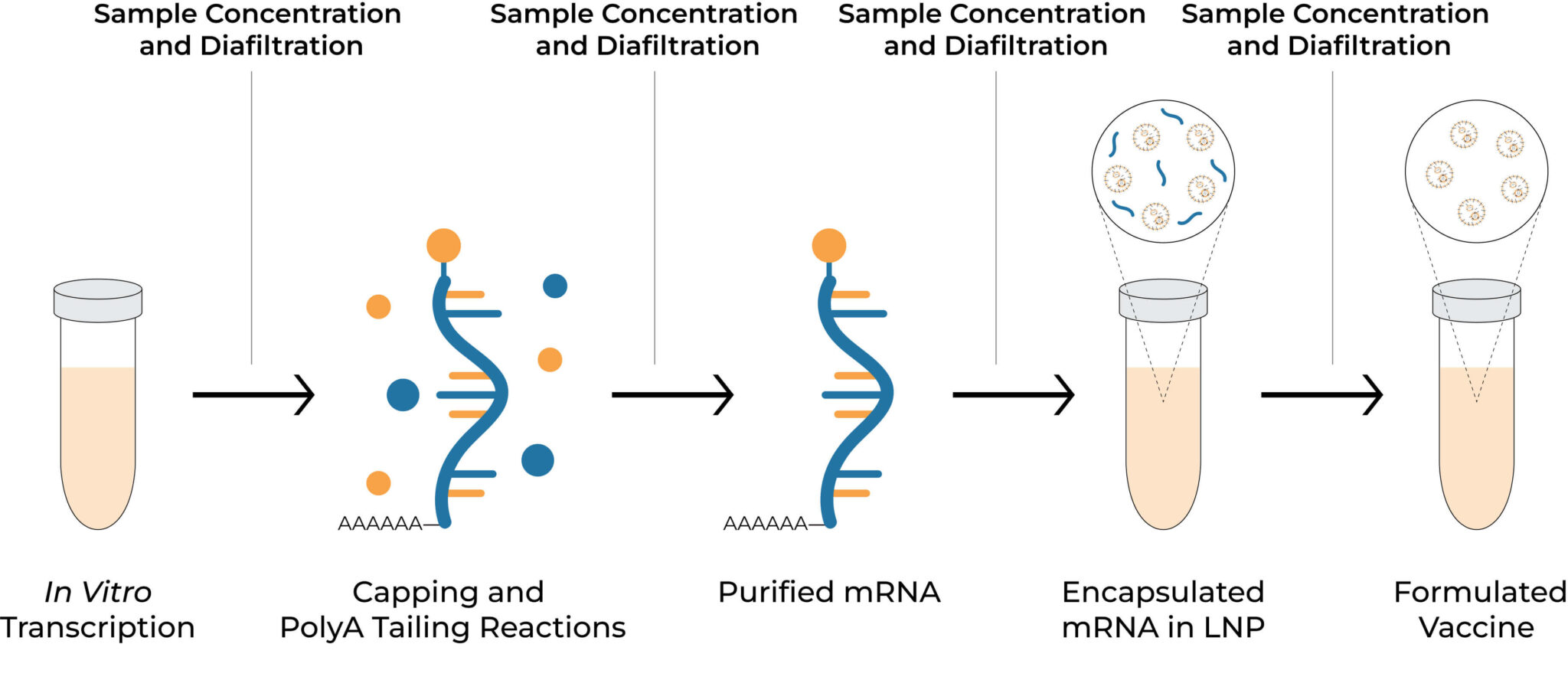
mRNA vaccine development workflow
When selecting an appropriate method for concentrating and formulating vaccines, several key considerations must be carefully balanced. Gentleness is crucial for preserving the structural integrity and immunogenicity of sensitive biomolecules. High yield is essential to ensure process efficiency and reduce waste. Scalability enables a seamless transition from lab-scale research to commercial manufacturing, while time and cost efficiency significantly influence the overall economic feasibility of vaccine production. Each of these factors influences the choice of formulation strategy.
Among the available methods, ultrafiltration stands out due to its superior performance in terms of gentleness and preservation of product quality. However, Dead-end filtration (DEF) using centrifugal units is prone to membrane clogging, leading to inconsistent results, low recovery, and restricted scalability.
| Considerations | Precipitation |
Column Chromatography | Evaporation | Lyophilization |
Ultrafiltration Dead-End TFF |
|---|---|---|---|---|---|
| Yield | |||||
| Gentleness | |||||
| Scalability | |||||
| Processing Time | |||||
| Cost Effectiveness |
Method for Vaccine Concentration and Diafiltration
In contrast, Tangential flow filtration (TFF) has become the preferred method for performing these steps due to its high filtration rates, gentleness, efficiency and scalability. However, the conventional TFF systems have large footprints, high hold-up volumes, and labor-intensive operation, making them inefficient for lab-scale workflows. Therefore, the introduction of a lab-scale TFF system addresses the need for process development and optimization, thus serving as a critical bridge between research and industrial application.
Vaccine Development Using the µPulse - TFF System
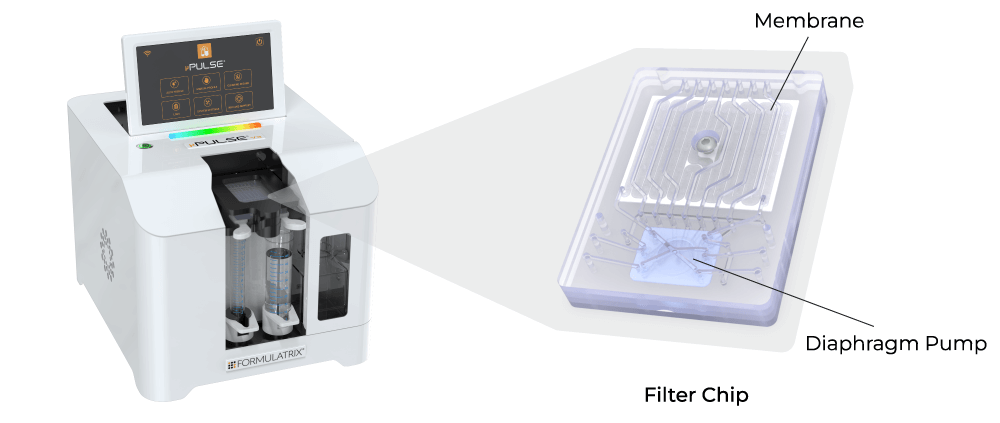
The Formulatrix µPulse® is an automated and miniaturized TFF system explicitly designed for lab-scale applications. It is well suited for the development of DNA, RNA, polysaccharide, and recombinant vaccines, while ensuring high product yield and quality. The entire fluid path is miniaturized on the filter chip by combining our patented microfluidic pumping technology with the TFF. This has reduced the hold-up volume to just 0.65 mL, ensuring maximum hold-up recovery.
Its customizable settings, including adjustable pressures and pump parameters, provide a gentle yet highly efficient process for vaccine development. The filter chips are available with modified polyethersulfone (mPES) and regenerated cellulose (RC) membranes that exhibit low fouling characteristics and are compatible with various sample types. Furthermore, it processes samples 4x faster than the dead-end centrifugal units and in a walk-away approach.
High-throughput Bioconjugate Cleanup with the aµtoPulse - TFF System
The aµtoPulse® is a fully automated, high-throughput TFF system with the world’s lowest hold-up volume of just 250 µL. It processes up to 54 samples per run, with up to 4 samples in parallel. Designed for flexibility, it handles starting volumes between 0.5 mL to 100 mL and can concentrate samples down to 250 µL with ±25 µL precision. The system supports up to four external buffer inputs or on-deck conical tubes, enabling automated multi-buffer diafiltration.
The advanced chip design with dual pumps delivers permeate flow rates up to 1.7x faster than the µPulse while minimizing shear, thus enabling the gentle formulation of vaccines. The chips are available with mPES (5–300 kDa) and RC (5–100 kDa) membranes, ensuring compatibility with a wide range of samples.
Each station offers independent regulation and monitoring of transmembrane pressure (0–32 psi), giving users full control over process gentleness and efficiency. The intuitive, browser-based software enables remote protocol setup, monitoring, and control, with secure data management compliant with 21 CFR Part 11 for GMP environments.
FAQs
What are the advantages of single-use filtration systems in bioprocessing?
Single-use filtration systems like the µPulse and the aµtoPulse offer clear advantages in bioprocessing by eliminating cross-contamination risk, reducing cleaning and validation burden, and accelerating turnaround time between runs. Because they do not require requalification, single-use systems simplify compliance in regulated environments and lower operational costs associated with water, chemicals, and labor. For sensitive biologics, nanoparticles, or cytotoxic materials, single-use filtration improves product safety, enables faster scale-up, and provides greater workflow flexibility, making it especially valuable for GMP, multi-product, and high-throughput bioprocessing applications.
Are there single-use TFF cassettes for small batch GMP production?
Yes, there are single-use TFF cassettes suitable for small-batch GMP production, and systems like the µPulse and the aµtoPulse are designed to work with disposable TFF chips that function as single-use filtration units. These single-use chips eliminate cross-contamination and help streamline compliance in regulated environments. This makes them ideal for small-batch, high-value biologic, nanoparticle, or viral vector production where safety, reproducibility, and regulatory traceability are critical.

