The Universal Need for Sample Concentration and Buffer Exchange
In nearly every corner of biotechnology, from developing antibody-drug conjugates and mRNA vaccines to purifying proteins, sample concentration and buffer exchange are fundamental steps. The quality and efficiency of these processes directly impact experimental outcomes, influence the development timelines, and determine the viability of the final product. For decades, tangential flow filtration (TFF) has been the gold standard for these workflows at the industrial scale.
The Lab-Scale Dilemma: From Industrial TFF to the Centrifuge
While TFF is the champion in pilot and manufacturing settings, conventional TFF systems are often impractical for the typical research lab. Their large footprint, significant hold-up volume (which leads to unacceptable product loss with small samples), and operational complexity make them unsuitable for the flexible, lower-volume needs of R&D.
This dilemma led the research community to adopt a different approach for lab-scale work: dead-end filtration (DEF), most commonly used in the form of centrifugal ultrafiltration units. Their small size, simplicity, and design for low volumes made them the de facto solution, a reasonable compromise for scientists who couldn't use a large TFF system.
The Hidden Costs of DEF
Anyone who has spent hours in the lab processing samples with centrifugal units knows this compromise comes with significant drawbacks. These conventional tools, while common, present critical limitations:
- Slow Filtration Rates: DEF, by its nature, forces liquid and solutes directly against the membrane. This causes rapid membrane fouling and concentration polarization, dramatically slowing down the filtration process and prolonging an already tedious task.
- Sample Loss and Aggregation Risk: The high concentration of molecules pressed directly onto the membrane surface, combined with the shear forces of centrifugation, can cause irreversible protein aggregation and denaturation. This results in lower yields of active, functional products.
- Labor-Intensive Operation: The dead-end units are hands-on, requiring manual interventions, careful sample handling, and often interrupting the process merely to track volume.
- Limited Scalability: The scalability of dead-end units for larger lab-scale quantities is restricted, often forcing users to run multiple units in parallel or engage in repetitive processing with the same unit.
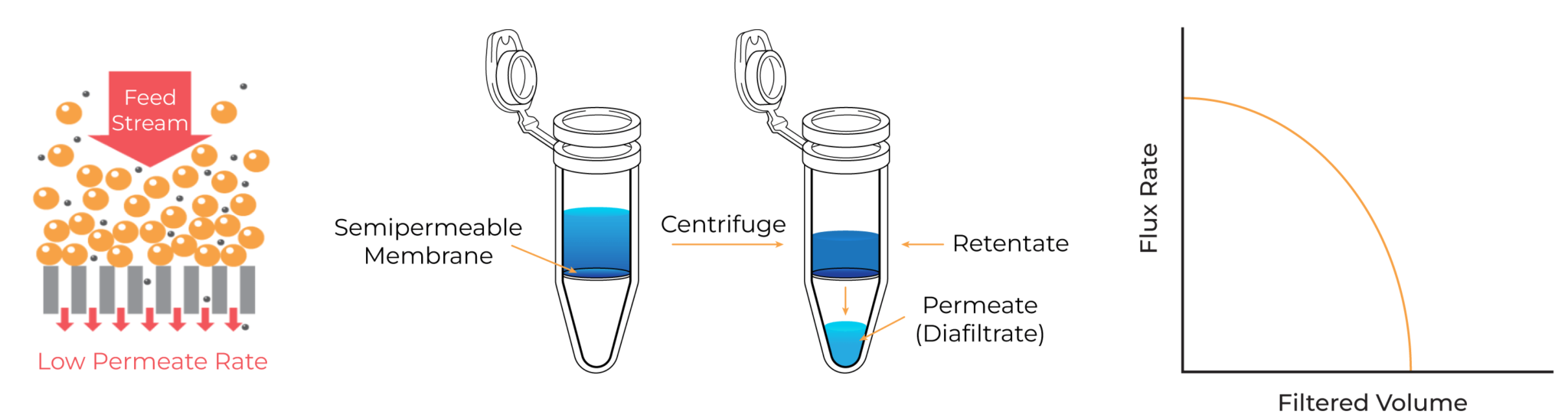
Envisioning the Ideal: TFF Performance in a Lab-Scale Format
This raises a crucial question: What if researchers didn't have to compromise? What would an ideal lab-scale system look like?
It would need to combine the best attributes of both technologies. It would have the high filtration rates and gentle processing characteristics of TFF, while also retaining the small-volume suitability and low hold-up volume that made dead-end filtration appealing in the first place. Such a system would offer faster processing, higher product recovery, reduced hands-on time, and valuable process data—all within a compact, benchtop-friendly format.
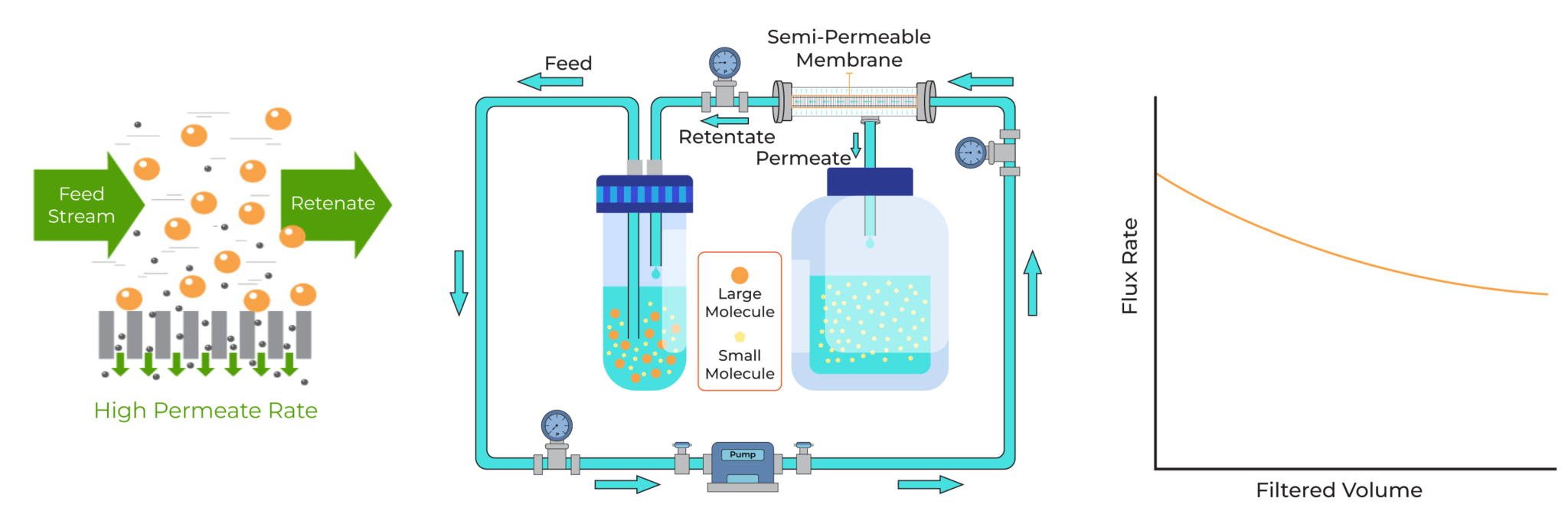
Tangential-Flow Filtration
The µPulse® - TFF System: Bridging the Gap
This ideal is now a reality. The µPulse - TFF System has been engineered to bridge this exact gap, delivering the power of TFF with the convenience needed for lab-scale work. It directly addresses the limitations of dead-end units by integrating these two worlds:
- Miniaturized TFF with Microfluidic Technology
The functional unit of the µPulse is the filter chip that has been designed by combining TFF with microfluidic pumping technology. This integration has reduced the hold-up volume to just 650 µL, which is up to 100% recoverable, ensuring minimal product loss for valuable and low-volume samples.
- Fast Sample Processing
The sample is constantly recirculated, and tangential flow keeps the membrane pores open, resulting in a permeate flow rate that is up to four times faster (4X) than that of dead-end centrifugal units.
- Automated Volume Tracking
The µPulse utilizes weight-based volume sensing for real-time, accurate volume tracking, eliminating the need for manual checks. By programming a target concentration factor or buffer exchange volume, researchers can trust the system to run unattended, freeing up valuable time while ensuring process reproducibility.
- Scalable Process
Whether you are trying to scale up or down, the µPulse allows you to control all the parameters of an industrial TFF at the research scale.
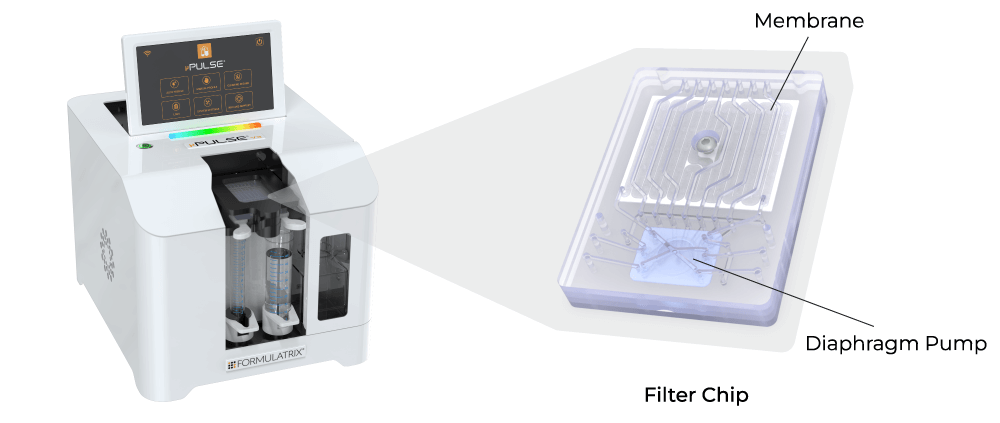
Conclusion
The µPulse system offers a clear evolution, replacing the slow, loss-prone, and manual nature of centrifugal devices with an automated, efficient, and gentle process that finally brings the true performance benefits of TFF to the research lab.
