Determine If Your Crystals are Protein or Not
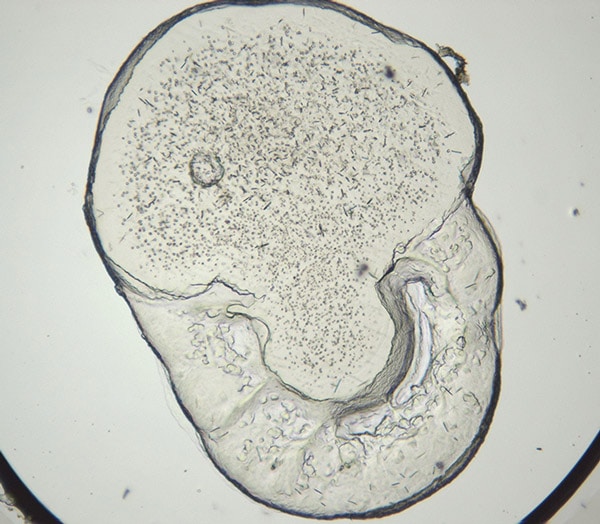
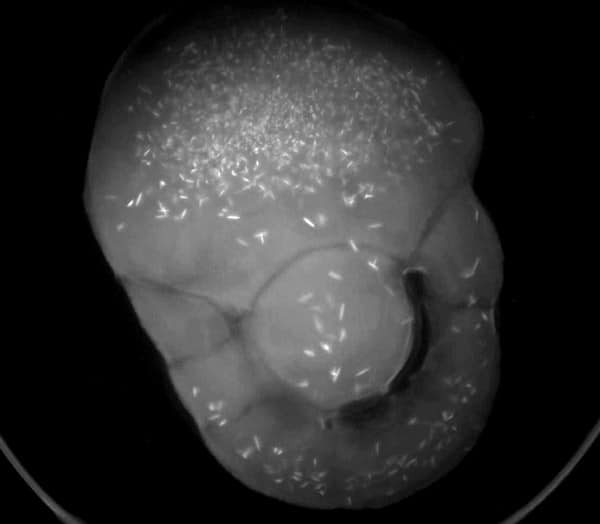
Protein crystallization drops are illuminated with ultraviolet (UV) light and the fluorescence generated from aromatic amino acids like tryptophan are detected to create an image. If no fluorescence is detected within a crystal, then it lacks enough aromatic amino acids to produce a signal and is likely not protein.
The UV imaging option is built into Rock Imager® with all several automated features, including automated imaging, extended focus imaging (EFI), and regions of interest (ROIs) that can help you see your crystals better.
The Rock Imager UV imaging solution uses 100% UV-optimized components: UV-grade optics, a UV-sensitive camera, and UV lighting. This optimization is critical for achieving the best image quality and fastest imaging speed possible.
Visualize Microcrystals with High Magnification Objectives
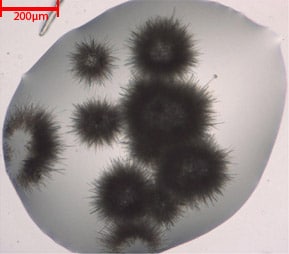
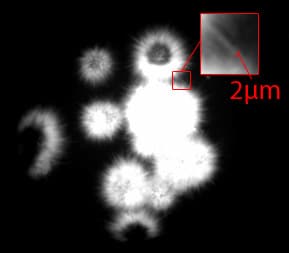
UV-optimized optics provide high-contrast images with the ability to see crystals as small as 2 μm. In the image to the right, individual protein crystal needles 2 μm in width are visualized with UV. The UV, LED and condenser lenses are positioned to maximize the intensity of the UV illumination in order to boost protein fluorescence signal strength. Also, the average RMS contrast (standard deviation of the pixel intensities) is optimized to increase the visibility of fluorescing crystals.
Both fixed and compound zoom options are available for Rock Imager UV. The fixed zoom option is available with one of the lenses listed in the table below. The compound zoom option includes two lenses on a motorized wheel.
| Objective | Numerical Aperture | Depth of Focus (mm) | Field of View (mm) | Pixel Size (µm) |
|---|---|---|---|---|
| 3.3x | 0.11 | 0.1 | 3.7 x 3.0 | 1.1 |
| 6.6x | 0.23 | 0.05 | 1.9 x 1.5 | 0.56 |
UV Optical Design that Preserves Visible Image Quality
Dual Light Path Option
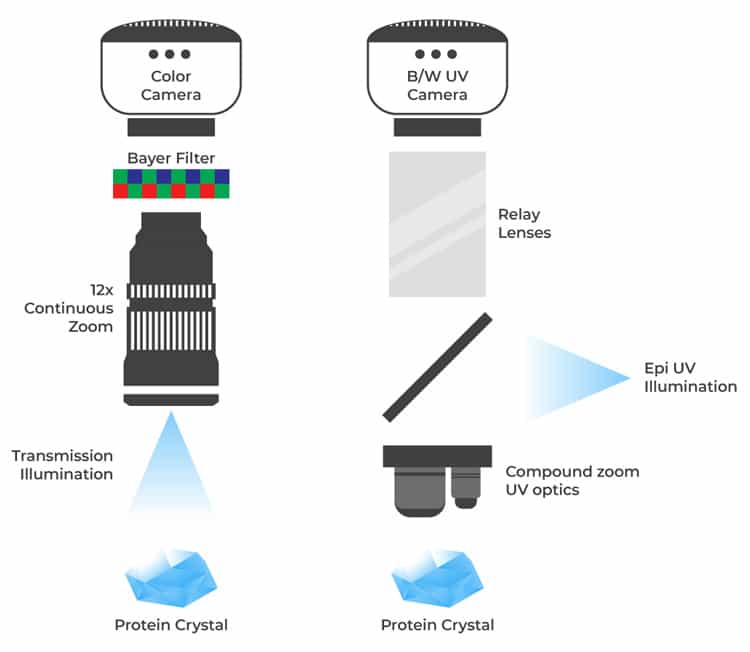
The color camera and 12x continuous zoom optics are incompatible with UV light, so colored imaging, continuous zoom and regions of interest are not available if visible and UV are combined into a single path. The FORMULATRIX solution uses two uniquely-optimized and adjacent lightpaths. The sample is moved from one microscope to the other with precise registration between the two images. The dual light path solution also corrects chromatic aberration that would not be possible with a single light path configuration.
UV Optical Design that Preserves Visible Image Quality
Dual Light Path Option

The color camera and 12x continuous zoom optics are incompatible with UV light, so colored imaging, continuous zoom and regions of interest are not available if visible and UV are combined into a single path. The FORMULATRIX solution uses two uniquely-optimized and adjacent lightpaths. The sample is moved from one microscope to the other with precise registration between the two images. The dual light path solution also corrects chromatic aberration that would not be possible with a single light path configuration.
Add UV Imaging on a Budget
Single Light Path Option
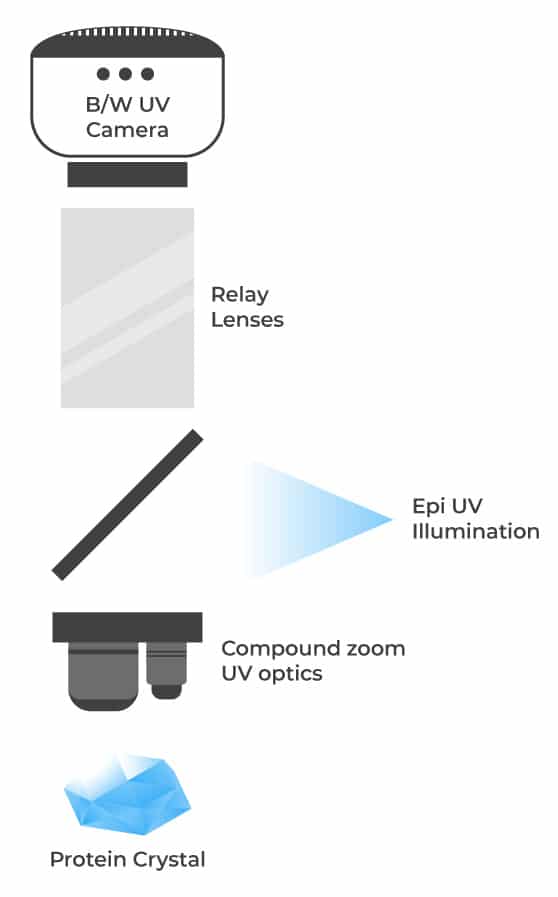
For a budget-friendly option, a single light path design is available where the visible optics and camera are shared with the UV optics. This option is only available with black and white imaging and fixed objectives.
For the Rock Imager 1, the optics for visible and UV imaging are shared and optimized to yield high-resolution UV images while retaining high optical quality visible images. A simple optical design is utilized to increase the throughput of UV fluorescence allowing for shorter exposure times while imaging. Two high numerical aperture objectives are utilized to image drops at varying magnifications.
FAQs
What is ultraviolet (UV) imaging in protein crystallization, and why is it important?
Ultraviolet (UV) imaging uses UV light to excite aromatic amino acids like tryptophan, producing fluorescence that helps identify protein crystals. Crystals lacking fluorescence are likely non-protein (e.g., salt crystals). This technique is valuable for distinguishing protein from salt crystals, detecting microcrystals, and enabling quick visual screening of crystallization plates.
Which amino acids fluoresce under UV light in protein crystallization (e.g., tryptophan, tyrosine, phenylalanine)?
Tryptophan, tyrosine, and phenylalanine are the three amino acid residues primarily responsible for the inherent fluorescence of proteins under UV light. However, tryptophan is much more fluorescent than either tyrosine or phenylalanine.
Why do some proteins fluoresce more than others under UV?
Fluorescence mainly depends on tryptophan concentration; the higher it is, the stronger the signal. However, plate media can dampen the signal or introduce noise, making proteins appear less fluorescent, which is why plate masking is important in UV imaging. Additionally, the drop fluid itself may absorb either the excitation UV light or the emitted fluorescence, further reducing signal strength.