Systems You Can Trust
Over 15 Years of Imaging Excellence
Used worldwide by the top 25 pharmaceutical companies and renowned academic centers.
Image Quality Second to None
Optimized Optics
Every imaging technology is precisely optimized for crystal clear images.
Increase Your Throughput
Automation Designed for Speed
Image entire 96-well plates in only 3 minutes and incubate up to 1500 plates.
Crystal Clear Imaging. Unparalleled Speed.
Rock Imager® - Crystallization Imagers are a series of automated imaging systems designed for protein crystallization screening. Capture superior-quality images of your protein drops while learning critical information about your crystals. Rock Imagers are available with various plate capacities and imaging features, so there is a model to fit every lab's budget and workflow.
Screen conditions with various imaging methods to discover more
Visible Light (Color), Cross-Polarization, UV Fluorescence, Multi-Fluorescence Imaging (MFI), Fluorescence Recovery After Photobleaching (FRAP), Second-Order Nonlinear Imaging of Chiral Crystals (SONICC).
Various models to fit your budget and workflow
Plate capacities from a single plate up to 970 SBS format plates or 1500 LCP plates.
Temperature regulation options available
Precise temperature control from 30°C down to 4°C.
Multiple plate options available
SBS, Linbro, Nextal, Terasaki/HLA and Lipid Cubic Phase (LCP) plate.
Seamless integration with Rock Maker - Crystallization Software
"We purchased the FORMULATRIX RI 54 imager a year ago and it has already proven to be irreplaceable for our scientists as each of them usually goes through thousands of experiments per day. ROCK MAKER / ROCK IMAGER software makes it much more convenient, saves time, and advances the projects faster. The quality of the images is very high. Even with the drop location mode, we can see every detail of the drop. Plus, having the UV imaging option aids well with filtering crystals for data collection. It's important to emphasize that FORMULATRIX customer support is exceptional. Any issue is addressed very quickly and efficiently."
Screen Conditions with More Confidence
See the Details of Your Drops
High optical resolution is achieved by increasing the numerical aperture of the objective. The trade-off, however, is that depth of field (depth of the image in focus at one time) is reduced. Rock Imager combines multiple slices into one Extended Focus Image (EFI) to allow the best of both worlds.
Capture Images the Way You Want
Multiple images of each drop can be captured with user-adjustable settings including exposure, polarization, and condenser aperture.
See Something Interesting? Zoom in for a Better Look
Specify regions of interest within a drop to zoom into. Rock Imager will automatically capture those areas in high resolution.
"Our Rock Imager 1000 has been a game-changer for our protein crystallization research. Its ability to continuously monitor experiments has significantly increased our productivity and yielded exceptional results. We've conducted over 1600 experiments in the past four years, and the equipment has proven to be highly reliable and robust. Its user-friendly interface has made it accessible to our entire research group."
Learn More About Your Drops
Multiple imaging technologies are available for Rock Imager allowing you to discover more about your protein drops:
Visible Light
The standard imaging technique for screening used by most crystallographers. Rock Imager provides high-resolution images.
Learn More
Ultraviolet (UV)
Determine if your crystals are protein or salt and find crystals in optically turbulent environments. Learn More
Multi-Fluorescence Imaging (MFI)
Find protein-protein complexes, increase crystal detection sensitivity, and incorporate UV imaging into your toolkit. Learn More
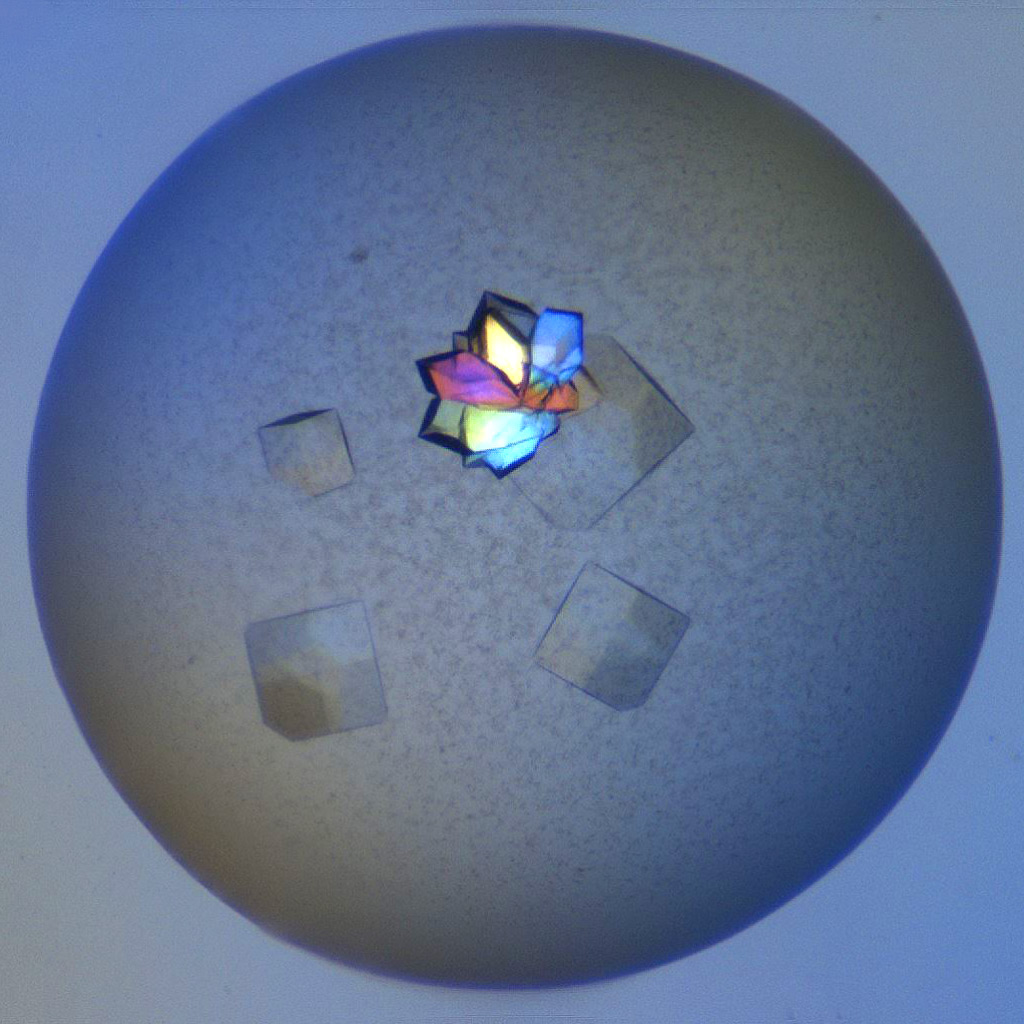
Second Order Nonlinear Imaging of Chiral Crystals (SONICC)
Detect microcrystals (<1 µm), determine which crystals are protein vs. salt, and increase crystal detection sensitivity. Learn More
Fluorescence Recovery After Photobleaching (FRAP)
Identify the optimal conditions for LCP crystal growth without having to wait on the crystals actually growing. Learn More
"The two ROCK IMAGERs in our Biochemical Institute at UZH have proven to be very valuable tools to advance our research in structural biology. The visible light and cross-polarization images in color and the highly focused UV fluorescence images, in combination with the sophisticated ROCK MAKER/ROCK MAKER Web software, are crucial as they allow researchers to efficiently sift through literally thousands of experiments. Vital to the success of these tools is the excellent response of the technical service team. Any hardware or software problems are addressed and solved in the shortest possible time."
Versatile Options for Any Lab
Models to Fit Your Lab's Throughput and Budget
With 4 different models all with flexible options, there is a Rock Imager to fit your lab's needs. Plate capacities range from a single-plate desktop model up to 970 SBS plates or 1500 LCP plates with hotel storage.
No Need to Change Your Microplate
All Rock Imagers are compatible with SBS, LCP, and microbatch plates. Options for Linbro and Qiagen EasyXtal plate compatibility are available.

Rock Imager 360 shows retrieving, imaging, and storing a plate
"As long-standing customers, we are very pleased with our Formulatrix system. The RI1000 and Formulator robots not only deliver reliability and robustness but also seamlessly integrate with the RockMaker software. This user-friendly platform simplifies workflow and project tracking across multiple research groups. The standout feature, however, is the exceptional customer service – the staff are always prompt, courteous, and efficient in resolving any issues. Formulatrix's dedication to continuous improvement and user satisfaction is evident in every aspect of their system. Highly recommended for anyone seeking reliability, efficiency, and excellent support."
Store More Plates in a Smaller Space
The Rock Imager 360 (RI360) variant can carefully incubate and capture superior-quality images of up to 364 microplates in a compact benchtop unit.
Key Benefits:
- Compatible with SBS-sized microplates and SBS-sized thin glass lipidic cubic phase (LCP) plates
- Precise incubation chamber temperature control from 30°C down to 4°C (chiller option is needed)
- Intuitive browser-based software allows users to access the program from any computer within a local intranet
- Know the status of your instrument instantly with a color-coded LED indication system
- Seamless integration with Rock Maker Crystallization Software
"The Rock Imager 360 has a small footprint but has enough capacity to manage our crystallization experiments. Like the RI1000 we have used, we are confident that this robust system will support our crystallization experiments for a long time."
See Every Detail of Your Drops with Superior Imaging Optics

Camera
- 5.0-megapixel, 2/3’’ color CMOS camera
- Captures high-quality images at up to 35 frames per sec
Optics
- 12x-motorized zoom optics with less than 1 μm feature resolution project sharp, high-contrast images to the camera and allows small-feature zooming
Lighting
- A Köhler light source with a motorized condenser provides high-contrast images with no sample heating and a reliable drop location
- Motorized top and bottom polarizers
Highest Resolution Visible Light Imaging
Fixed Objective Option
Two objectives are available for imaging protein drops. The large field of view objective is used for locating drops with two high magnification objectives available for high-resolution imaging. The software automatically determines the best objective to use based on the size of the drop.
Continuous Zoom Optics
Infinite zoom values are available up to 12x magnification, allowing you to zoom in exactly to drop sizes and Regions of Interest (ROI).

"We have had our ROCK IMAGER 1000 for nearly 2 years and have setup and imaged >7000 plates in that time. The system has performed very reliably over that span from a mechanical standpoint and the associated Rock Maker software has made tracking, viewing and organizing our many projects simple and easy. The seamless integration of our Formulatrix Formulator with the imaging system has allowed us to carry out optimizations in a high throughput manner as well. Just as important is that the service and support have been excellent, with most issues dealt with remotely and new customer-driven features added at regular intervals."
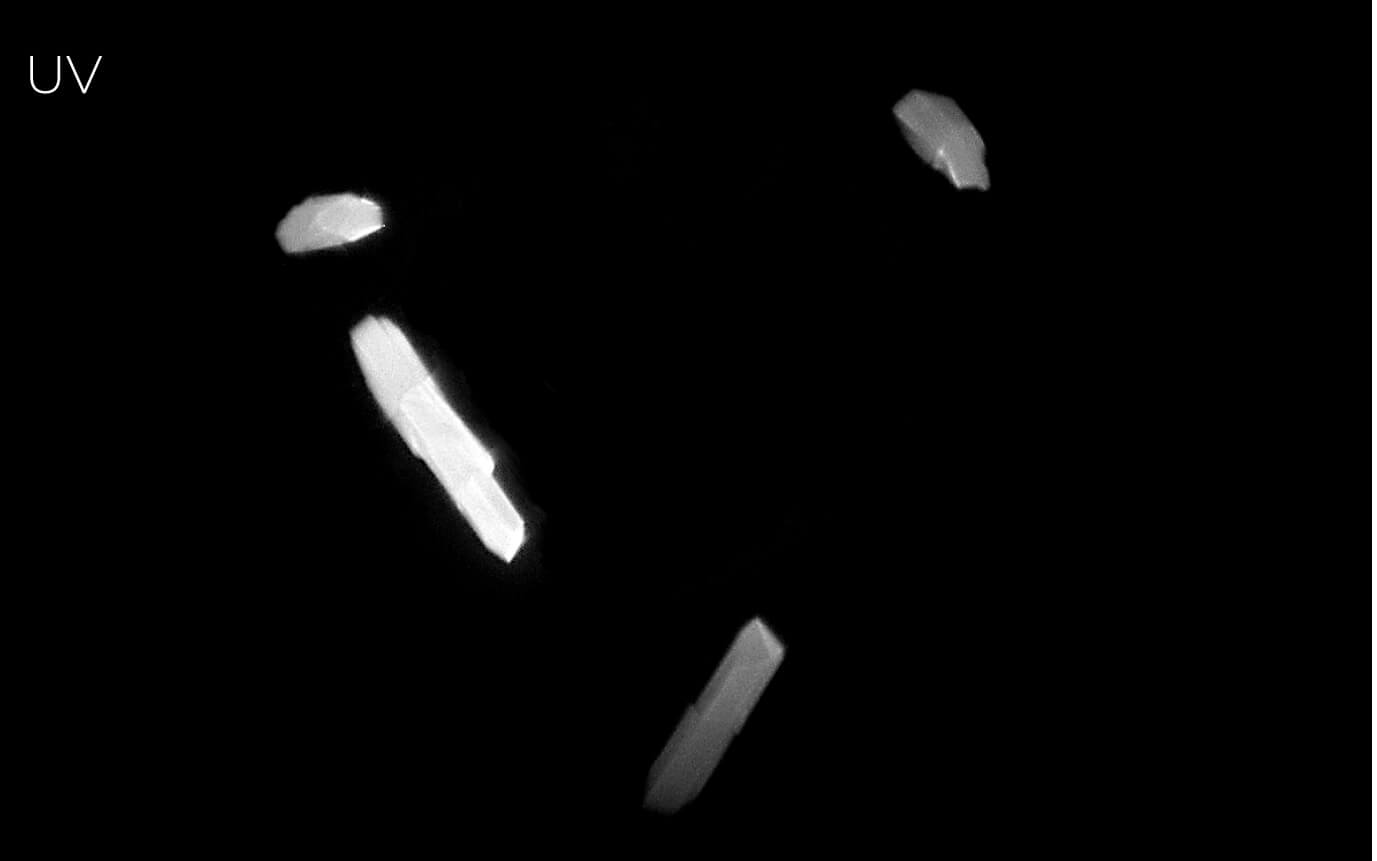
Detect Crystals with Ultraviolet Imaging (Optional)

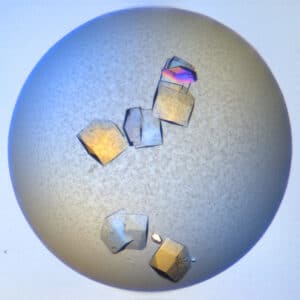
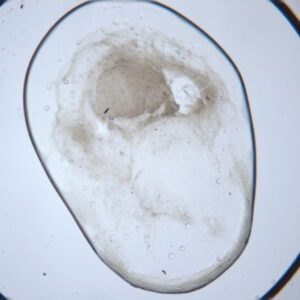
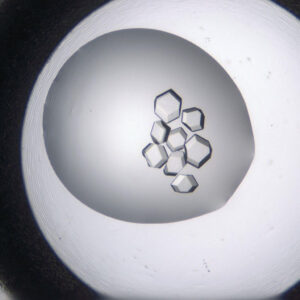
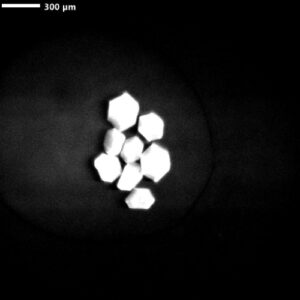
Visible Light - Protein Crystals

UV - Protein Crystals
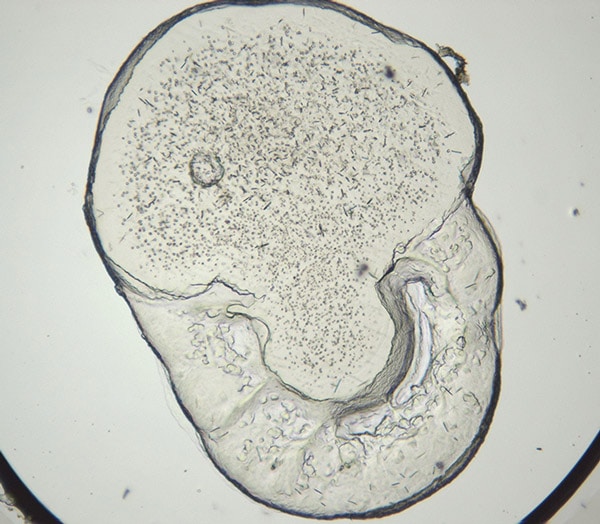
LCP - Visible Light
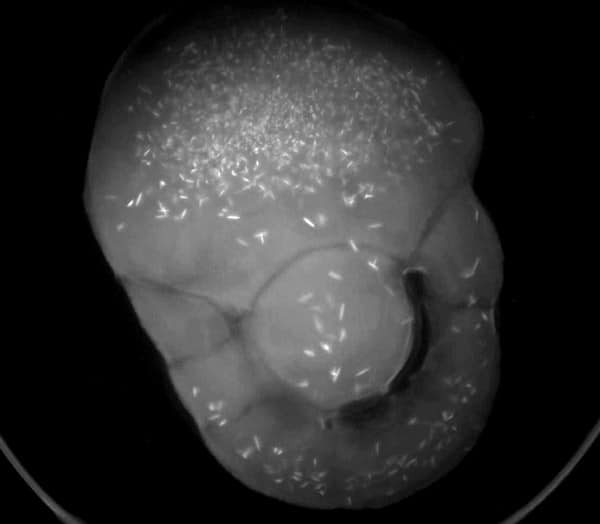
UV - LCP
Protein crystallization drops are illuminated with ultraviolet (UV) light, and the fluorescence generated from aromatic amino acids like tryptophan is detected to create an image. If no fluorescence is detected within a crystal, then it lacks enough aromatic amino acids to produce a signal and is likely not a protein.
The UV imaging option is built into Rock Imager with all several automated features, including automated imaging, extended focus imaging (EFI), and regions of interest (ROIs) that can help you see your crystals better.
The Rock Imager UV imaging solution uses 100% UV-optimized components: UV-grade optics, a UV-sensitive camera, and UV lighting. This optimization is critical for achieving the best image quality and fastest imaging speed possible.
"We buy a lot of products for FORMULATRIX such as ROCK IMAGER (both 4c system and RT system), FORMULATOR and NT8 for protein crystallization when using this machine we do face some problems and once I even want to return them back but people from FORMULATRIX always try their best to solve the problems. Finally, I think FORMULATRIX is really a responsible company and they always try their best to serve the customers. More important, the products are easy using."
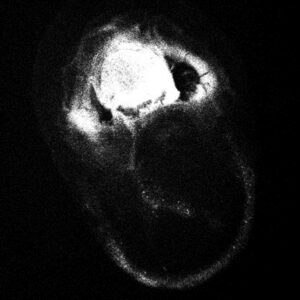
Find Protein-Protein Complexes With Multi-Fluorescence Imaging (Optional)
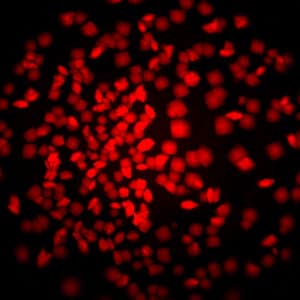
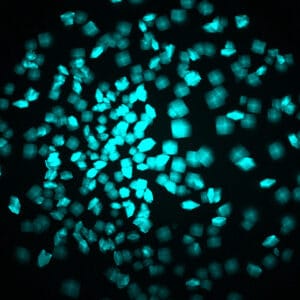
With MFI, you can now differentiate between crystals of a protein-protein complex and crystals of just one protein. Simply, label the two proteins, or subunits, prior to crystallization with two different amine-reactive dyes and then image your drop at the two corresponding wavelengths. Crystals that fluoresce at both wavelengths are protein-protein complexes, whereas those that fluoresce at only one wavelength are of a single protein.
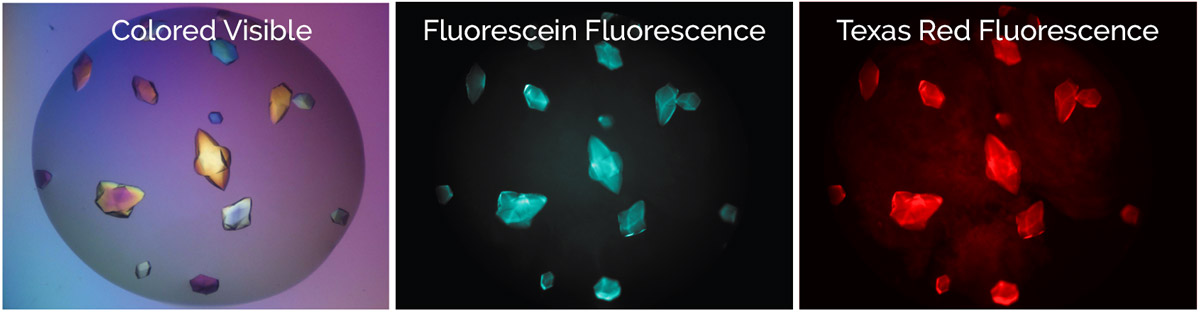
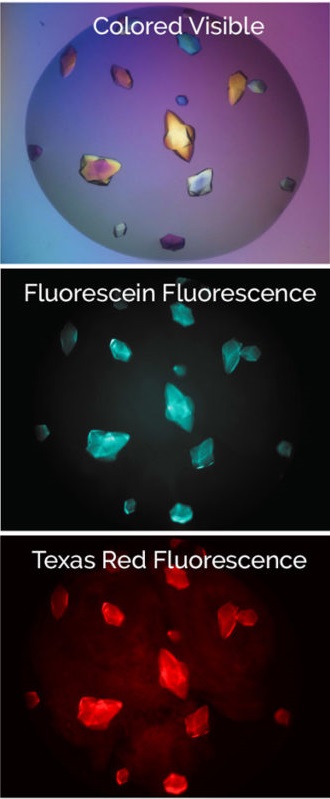
Bovine pancreatic trypsin inhibitor (BPTI) labeled with Fluorescein and trypsin labeled with Texas Red.
Crystals fluoresce at both wavelengths indicating that they are the BPTI-trypsin complex.1
"Within our structural biology facility, we have already used the Rock Imager 1000 for over a decade and are very enthusiastic about it. Recently, we purchased a new system to meet our demands. The easy-to-use system, user-friendly software, and high-quality camera with UV option are the reasons why we rely on Formulatrix products for our high-throughput crystallization experiments. The Rock Imager 1000 is reachable from everywhere via the Rock Maker Web software, which even allows analyzing crystallization plates when working at home."