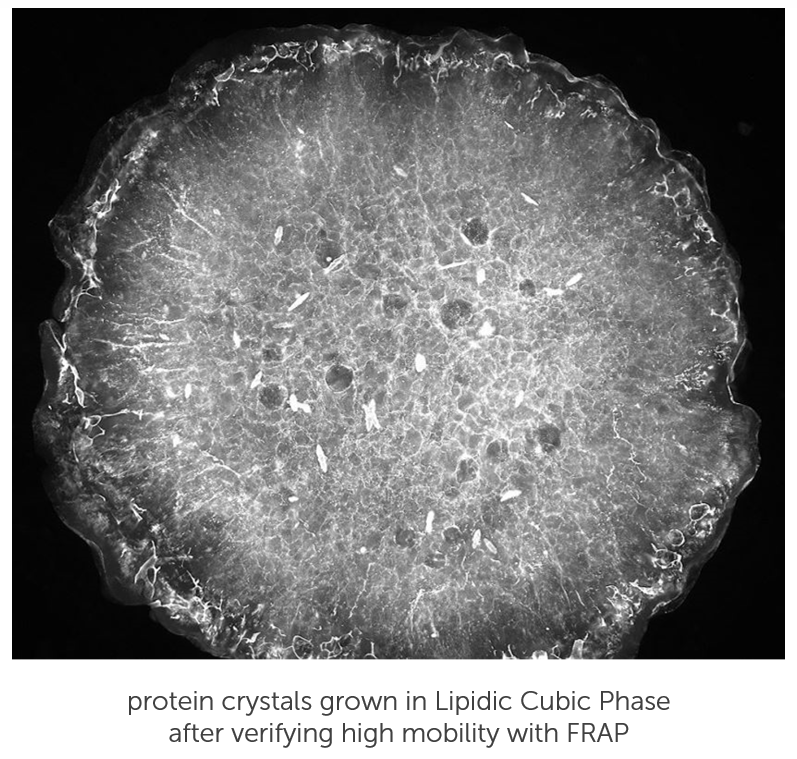
Prescreen for LCP Crystal Growth
High protein mobility and fast diffusion rates correlate well with crystallization conditions.
Save Time + Money
Focus your research on those conditions conducive to LCP crystal growth.
Convenient + Easy-to-Use
Initiate a complete analysis of your plate with a few clicks and store all your data in one spot.
Find the optimal conditions for LCP crystal growth, without waiting on your crystals.
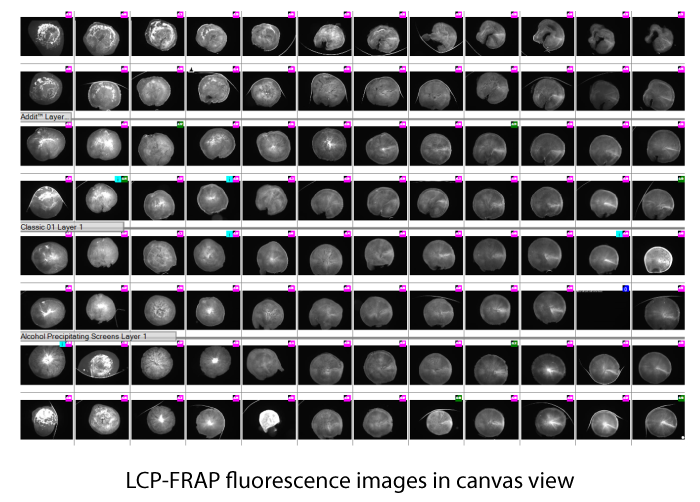
Prior to setting up crystallization experiments, you can quickly screen Lipidic Cubic Phase (LCP) conditions using Fluorescence Recovery After Photobleaching (FRAP). FRAP is an optical technique that assesses how easily protein is moving about in an LCP drop. If protein is aggregated, or the LCP structure is collapsed, the protein will not diffuse and therefore never crystallize. FRAP is a great tool to accelerate crystallization methods and rule out suboptimal conditions without having to wait on the crystals actually growing.
The automated FRAP imaging system was designed specifically for performing the FRAP assay on 96-well LCP crystallization setups. The entire system is automated and user-friendly requiring a few clicks of a button to initiate a complete analysis of your plate. All of the results and analyses are integrated into FORMULATRIX' protein crystallography specific software lab management software (ROCK MAKER and ROCK IMAGER).
FRAP was developed in collaboration with Vadim Cherezov (TSRI) and the JCIMPT center led by Ray Stevens and supported by the NIH Common Fund in Structural Biology.
Prescreen Crystallization Conditions
One of the main factors for successful LCP crystallization is the ability of the protein to diffuse within the lipid bilayer. The diffusion rate of the protein is influenced by protein aggregation, structural properties of the LCP, and the chemical environment.
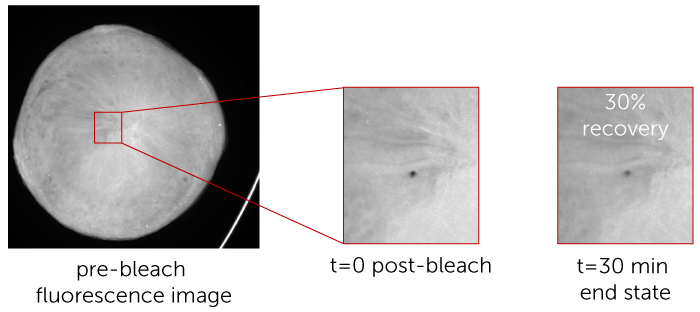
The diffusion rate can be determined by FRAP, which measures the amount of time required for the fluorescence intensity of a tagged protein to reestablish itself within a small area in the LCP drop that has been subject to optical bleaching.
Using FRAP, you can screen your crystallization experiment in under 50 minutes to determine the mobile fraction of your protein and whether or not that condition is conducive to forming protein crystals.
Data Collection Options
High Throughput
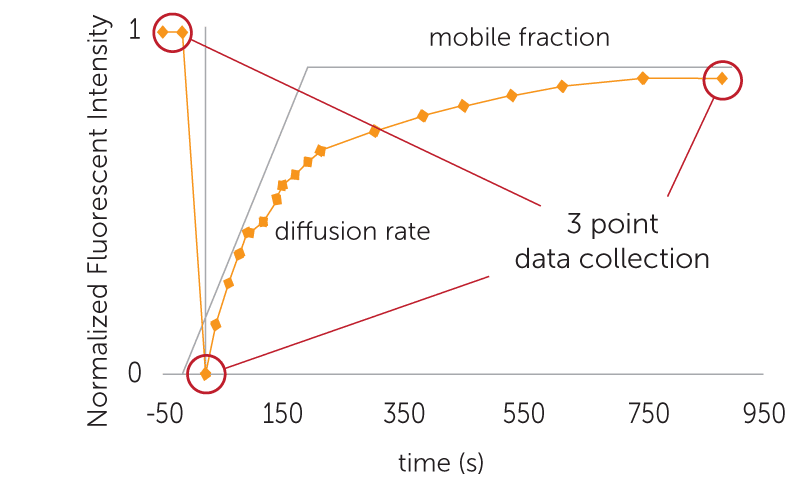
Each complete LCP-FRAP process takes about 15-20 minutes. To increase throughput, the system can be set to collect only the end state fluorescence intensity.
Only mobile fraction results are measured in this high throughput mode, which provides researchers the information needed to identify positive conditions. Most often, a mobile fraction >0.20 (20%) is a good threshold for a positive condition. In this mode, you can collect mobile fraction data on an entire 96-well plate in under 50 minutes.
Complete Recovery
Drops imaged using the complete recovery setting will be photographed many more times following the bleach time (time zero) in order to more closely capture molecular diffusion and generate a single- or double-component Bessel function curve.
Single-component recovery curves are enough for most experiments to analyze a protein´s diffusion rate and recovery time. However, if the experiment has two different types of molecules diffusing, such as labeled lipids, then a double-component Bessel function curve is more appropriate.
Data Collection Options
High Throughput:
Each complete LCP-FRAP process takes about 15-20 minutes. To increase throughput, the system can be set to collect only the end state fluorescence intensity.
Only mobile fraction results are measured in this high throughput mode, which provides researchers the information needed to identify positive conditions. Most often, a mobile fraction >0.20 (20%) is a good threshold for a positive condition. In this mode, you can collect mobile fraction data on an entire 96-well plate in under 50 minutes.
Complete Recovery:
Drops imaged using the complete recovery setting will be photographed many more times following the bleach time (time zero) in order to more closely capture molecular diffusion and generate a single- or double-component Bessel function curve.
Single-component recovery curves are enough for most experiments to analyze a protein´s diffusion rate and recovery time. However, if the experiment has two different types of molecules diffusing, such as labeled lipids, then a double-component Bessel function curve is more appropriate.
Fast and Easy Data Review
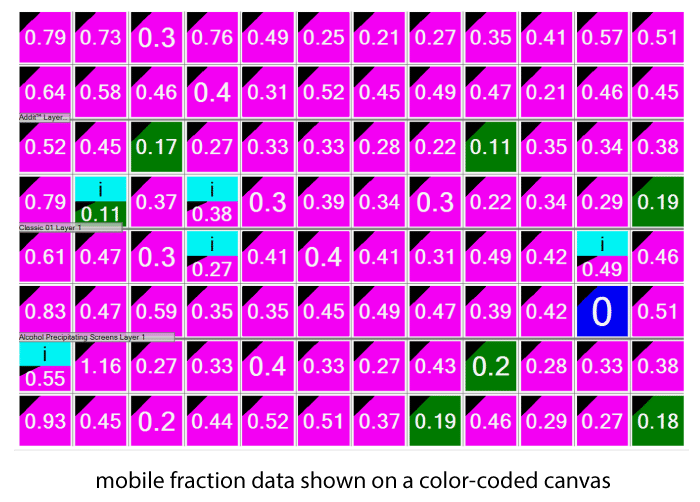
FRAP automatically calculates the percent mobile fraction for each drop. The mobile fraction score can then be related to the ability of the protein in that condition to form protein crystals. The higher the mobility, the greater the chance for crystallization. Scores are displayed in a color-coded overview allowing users to identify positive wells at a glance.
Models to Fit Your Workflow and Budget
FRAP comes in two configurations to better fit your lab's needs:
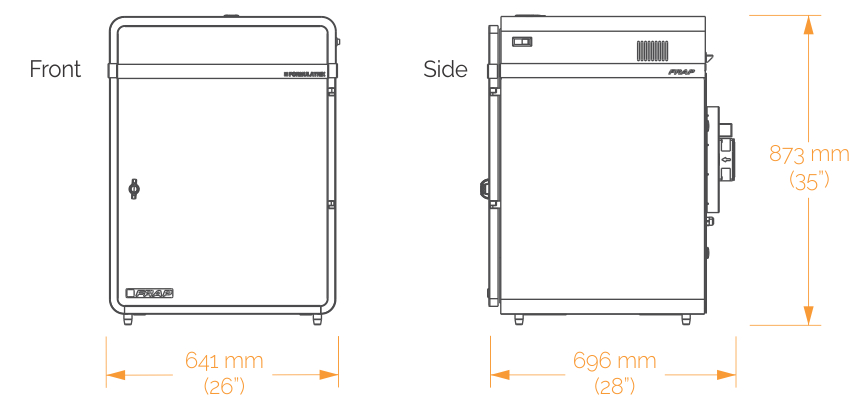
A benchtop model capable of imaging two plates at a time. Best for lab's where throughput is not a concern.
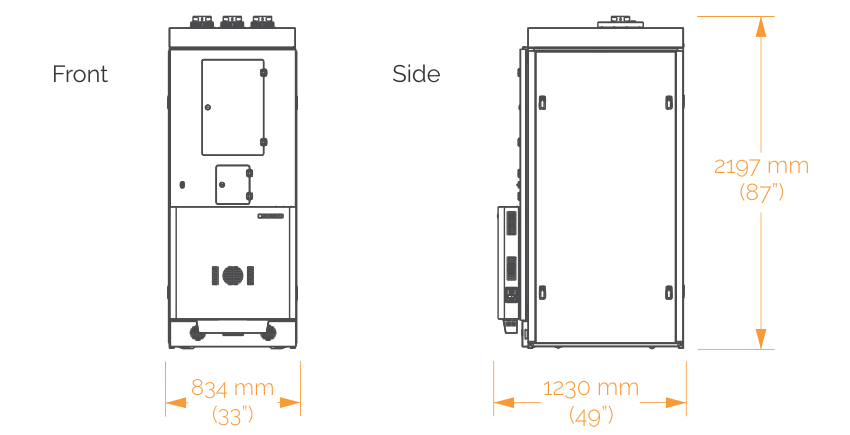
An integrated model that is built into a ROCK IMAGER 1000 which allows you to store and automatically image up to 970 plates. Two plates can be imaged at the same time - one with visible light and one with FRAP.