Protein concentration and buffer exchange steps are integral to any preparative scale protein production, whether for structural biology, or general biochemistry needs. These steps must be done at least once at the end of the purification protocol, or even between different chromatographic steps, adding up to a significant amount of time.
Current Methods
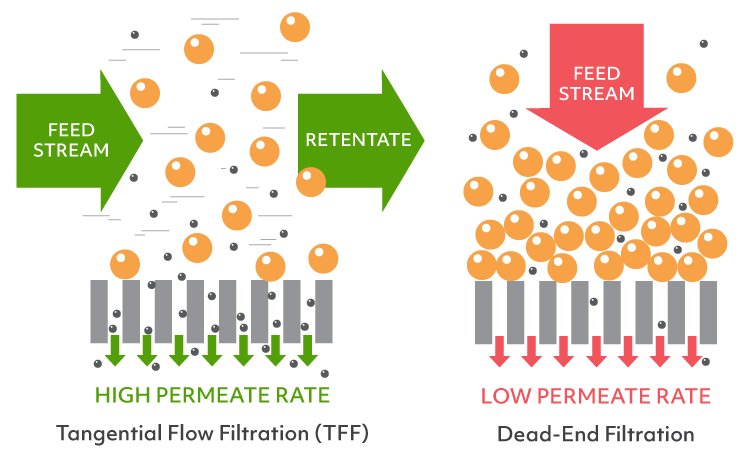
Ultrafiltration is the current standard for concentrating proteins and, on lab scale, typically involves disposable centrifuge-driven devices. These devices are dead-end in nature (the flow is perpendicular to the membrane) and create a concentration gradient at the membrane. The concentration at the membrane can far exceed the target slowing down the flow of the filtrate and increasing material loss due to aggregation/precipitation. The only way to monitor the concentration progress or to mix the sample is by periodic interruption of the centrifugation, making it a fairly hands-on method.
Membrane dialysis is the most popular buffer exchange method also involving a molecular weight cutoff membrane driven by the osmotic pressure. While being a hands-off method, it requires a large excess of the dialysis buffer, a long dialysis time (8-12 hours), and a subsequent concentration step.
On an industrial scale, both buffer exchange and concentration are accomplished with a single device employing tangential flow filtration (TFF). In addition to the filtrate flow across the membrane, TFF systems will also have an active flow parallel (or tangential) to the membrane. This flow mixes the sample (retentate) in real-time, drastically reducing the concentration gradient and allowing for filter dialysis (diafiltration) if the desired buffer was added to the retentate. However, even the smallest of the TFF devices do not fit in the typical lab workflow having the dead volume in tens to hundreds of milliliters.
µPULSE TFF System
Here we present our miniaturized TFF system, one of the smallest device of its kind available, intended specifically for lab-scale purifications. It has a completely recoverable dead volume under one milliliter and can accommodate volumes up to 50 mL if performing diafiltration in the same run, or up to 100 mL if only concentrating. The µPULSE TFF System is capable of ultrafiltration rates up to 5 times faster compared to a 15 mL centrifugal device and is completely automated with non-contact liquid sensing, bringing the retentate volume down to the specified setpoint.

The µPULSE TFF System features a consumable cartridge that encapsulates a microfluidic pump and a slightly larger membrane than a typical 15 mL centrifugal device (~7.1 cm^2 compared to 12.4 cm^2) to allow for faster sample concentration.
| Millipore Amicon Ultra 15 | FORMULATRIX® µPULSE TFF | |
|---|---|---|
| Permeate Flow Rate (mL/min)* | 1.2 | 2.9** |
| Membrane Flux (mL/cm/min)* | 0.16 | 0.24 |
| Protein Recovery | <85% | 90-99%* |
| Min – Max Volume | 0.2 – 15 mL | 0.4 – 50 mL (0.4 – 100 mL)*** |
| Max Concentration | 75x | 100x |
Notes:
* Conducted with 1 mg/mL BSA
** FORMULATRIX® membrane size is 60% larger than Amicon
*** 100 mL possible by filling both tubes with protein solution
Concentrate Samples Faster with TFF
Unlike centrifuges with traditional dead-end filtration systems, the µPULSE TFF System prevents a high concentration gradient from forming at the filtration membrane. This means no reduction in the permeate flow rate and increased sample recovery. With TFF, the concentration process is continuous and gradual allowing for samples to be concentrated over 5x faster than using dead-end filtration.
The µPULSE TFF System utilizes a larger filtration membrane (~60% larger) as compared to traditional dead-end filtration systems. This larger membrane boasts a higher membrane flux allowing you to concentrate samples faster so you can move on with your research.
Simple Diafiltration (Buffer Exchange, Desalting)
Along with concentrating your sample, there is an option for automatic diafiltration to facilitate buffer exchange or desalting processes. Set up is easy with two 50 mL centrifuge tubes located at the front of the system: one tube holds your sample solution, the other a buffer, or any other solution of your choice. The system can be set in a continuous diafiltration mode or set for discrete diafiltration steps. The status of the diafiltration can be monitored through the web-based software application.

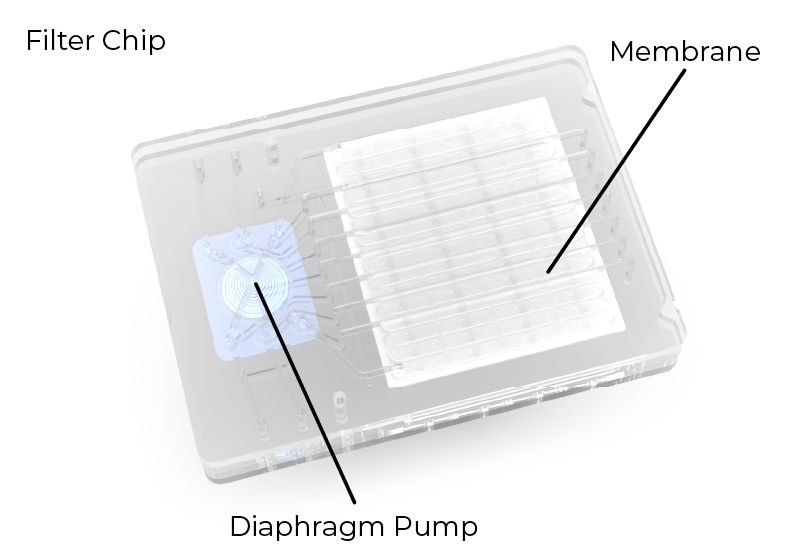
Easy to Maintain with Disposable Filter Chips
The µPULSE TFF System uses disposable filter chips that make it easy to keep the system clean and prevent any cross-contamination. A built-in diaphragm pump switches between vacuum (-10 - 0 psi) and pressure (40 - 50 psi) to circulate solutions over the concentration membrane. The chip and source reservoirs are also pressurized (15-20 psi) to help force permeate out and expedite the process. Four different concentration membranes are available with cutoff sizes of 10kDa, 30kDa, 50kDa, and 100kDa to cover a wide variety of protein sizes.
Reusability and High Efficiency
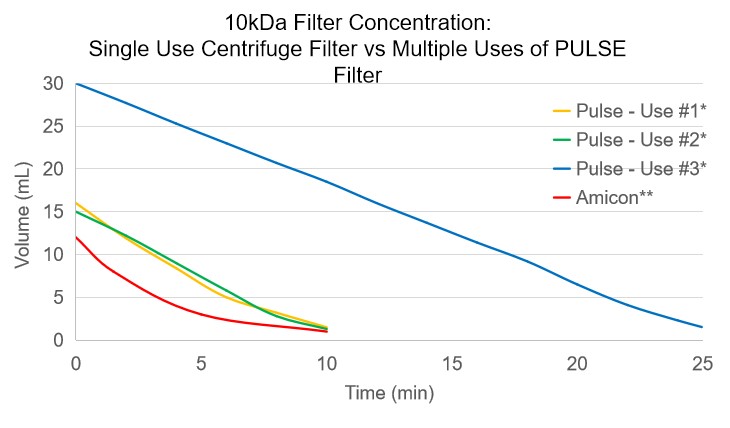
The PULSE TFF consumable can be reused multiple times with almost no loss in efficiency. Where standard centrifuge filters have an exponential decay in concentration rate the PULSE TFF consumable concentrates at a steady pace, only slowing when extremely high concentrations are met.
Even when concentrating a protein 6x larger than the cutoff size, the PULSE TFF consumable outperforms the standard centrifuge tube multiple times over, easily concentrating 5-10 times as much solution before needing to be replaced.
Notes:
*Conducted with 1mg/mL BSA (~66kDa)
**Conducted with 1mg/mL Cytochrome c (~12kDa)
