Authors: Ellen Gualtieri1, Eugene Chun2, Wei Liu2, Lance Ramsey1
1Formulatrix, Inc. 2Arizona State University
Problem:
Finding the optimal conditions to crystallize integral membrane proteins is time consuming. Crystals can take two weeks or more to form making it a lengthy process in optimizing growth conditions.
Fluorescence Recovery After Photobleaching (FRAP)
Prescreens crystallization conditions to eliminate those conditions that are not conducive for crystallization. In just 30 minutes one can narrow down to a small subset of conditions that are amenable to crystallization.
If proteins can not diffuse in the lipidic cubic phase,
then it is not possible for them to form crystals.
Save time by checking protein preparation with FRAP
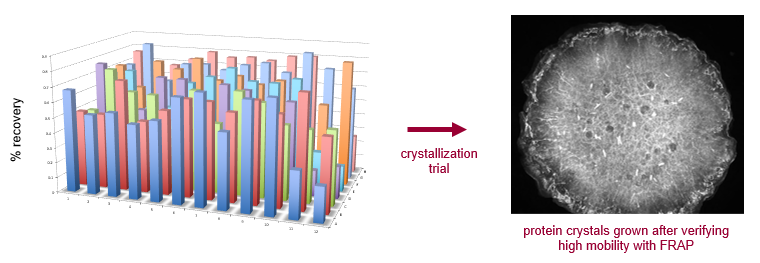
FRAP was carried out over a broad range of salt conditions on a 96-well plate for an integral membrane protein. Good bleach recovery in the majority of conditions suggests it is worthwhile to pursue LCP crystallization with this sample.
FRAP can determine which protein preparations are best
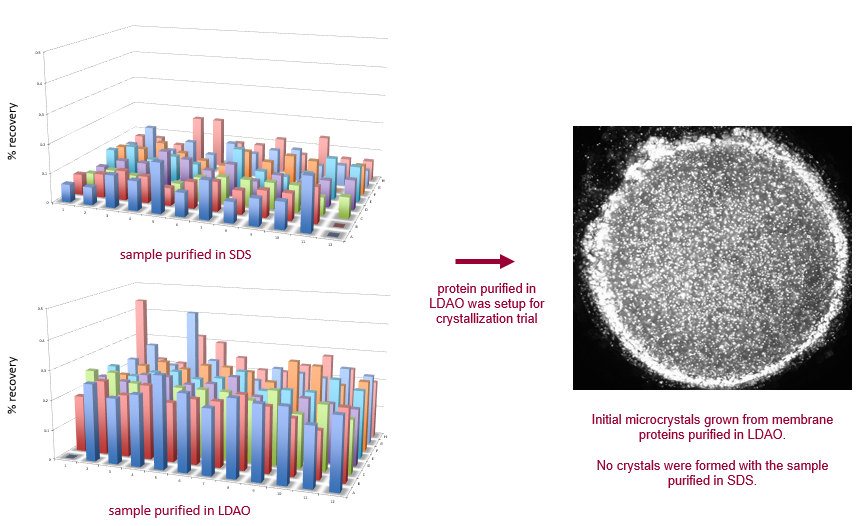
An integral membrane protein was purified in two different detergents, resulting in significant differences in FRAP recovery among a broad range of crystallization conditions. LDAO is shown here to be a much more favorable detergent for purification, and should be used for crystallization trials. The protein purified in SDS has little to no mobility, indicating that it would not crystallize.
Screen 96 conditions in 30 minutes
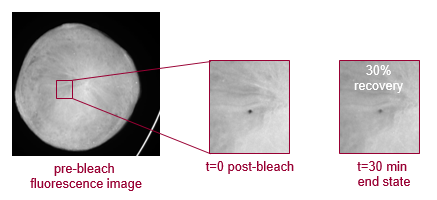
FRAP automatically analyzes 96 well LCP plates to determine percent mobile fraction for each drop. Each well is bleached by a focused laser spot, and the recovery of fluorescence after a fixed time point is measured. The percentage of proteins that are mobile in the drop are indicative of whether or not crystallization may occur.
1. The FRAP imager emits a photobleaching laser pulse which bounces off a dichroic mirror, through an excitation filter, and off a 2nd dichroic mirror, bleaching the sample.
2. A LED shines light through a condenser and the light follows the same path as the laser, illuminating the sample.
3. Fluorescent images are then processed to determine the recovery time of the photobleached spot indicating the protein’s mobility.
Easy Data Review
A software suite is used to track and analyze scores for each experiment. Scores are displayed in a color coded over-view allowing users to identify positive wells at a glance. Users can mark positive wells in the software for future reference. In the above example, well H8 has the highest mobile fraction rate and is most likely to grow crystals. Well D12 has no mobile fraction and is the least likely to grow crystals.
Reference
Yin, X.; Xu, H.; Hanson, M.; Liu, W., GPCR crystallization using lipidic cubic phase technique. Current pharmaceutical biotechnology2014, 15 (10), 971-979.