Viruses serve as invaluable tools in scientific research, allowing for the study of fundamental biological processes like gene expression and protein synthesis. Adeno-associated viruses (AAVs), lentiviruses, and bacteriophages are widely used as vectors for gene therapy due to their ability to integrate into the host genome. Similarly, virus-like particles (VLPs), non-infectious nanoshells that mimic the structure of viruses, are highly immunogenic and used as vaccines against various diseases. They can also be engineered to carry therapeutic agents for drug delivery and used as building blocks for nanomaterials and nanodevices.
Virus production workflows involve several stages, including propagation, harvesting, purification, and formulation. The process begins with the cultivation of virus-infected host cells (mammalian, insect, or bacterial cells). Once propagated, the viral particles are then harvested from culture medium or host cell debris. It is followed by purification to remove contaminating proteins, nucleic acids, or media components. Finally, the purified viruses are formulated for storage or downstream application, necessitating sample concentration and diafiltration (or buffer exchange). These processes are also required at the harvesting stage for partial purification of the propagated viruses.
VLP production and formulation workflow
Several factors must be considered when selecting a method for virus harvesting and formulation. Gentleness of the process is essential to ensure the quality, stability, and yield of the final product. Additionally, the method must be scalable from laboratory research to industrial-scale manufacturing, which involves balancing cost-effectiveness with high productivity. Time efficiency is also critical, as delays in processing can result in lower yields and higher costs. The table below outlines the typical methods employed for virus harvesting and formulation.
| Considerations | Centrifugation | Chromatography | Precipitation | Ultrafiltration |
|---|---|---|---|---|
| Gentleness | ||||
| Scalability | ||||
| Yield | ||||
| Time Efficiency | ||||
| Cost Effectiveness |
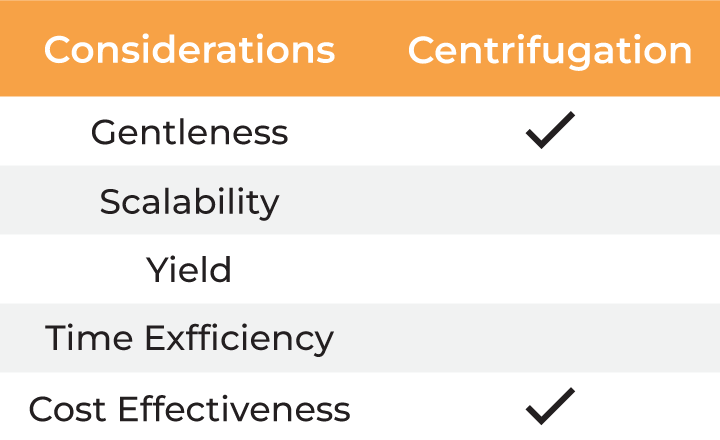
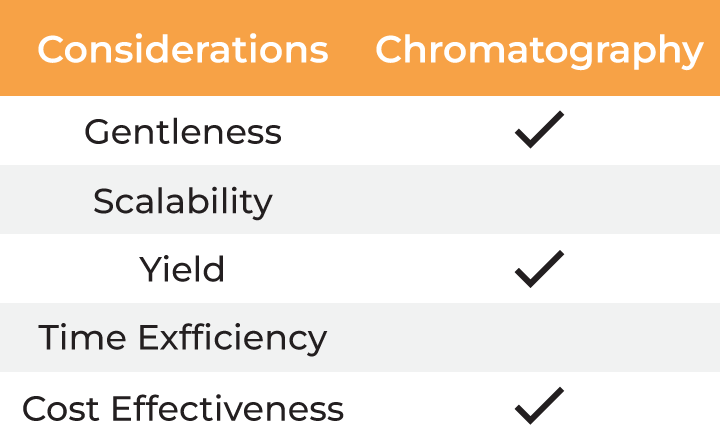
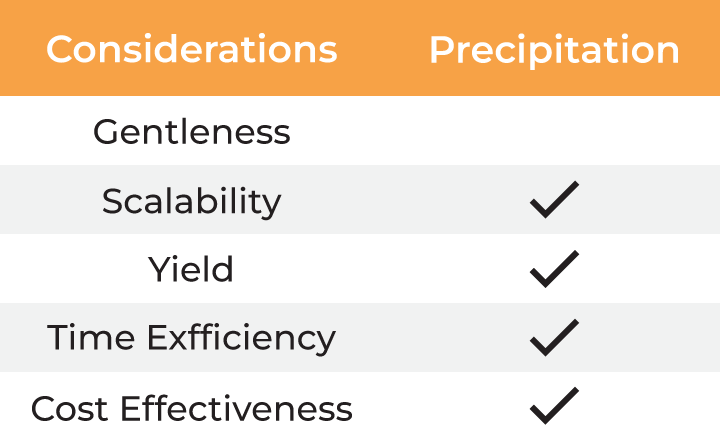
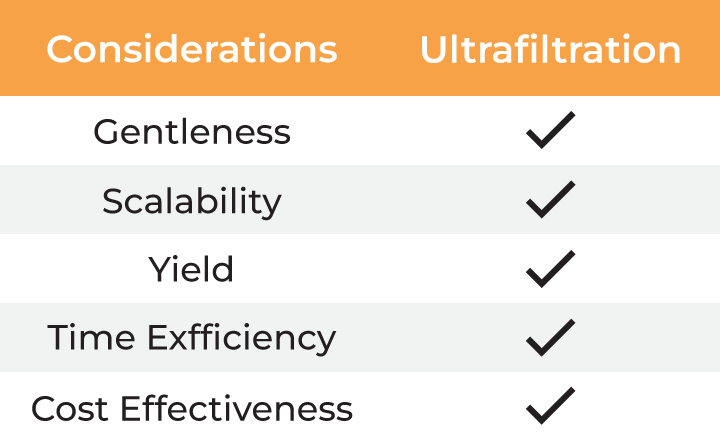
Table: Methods for virus harvesting and formulation
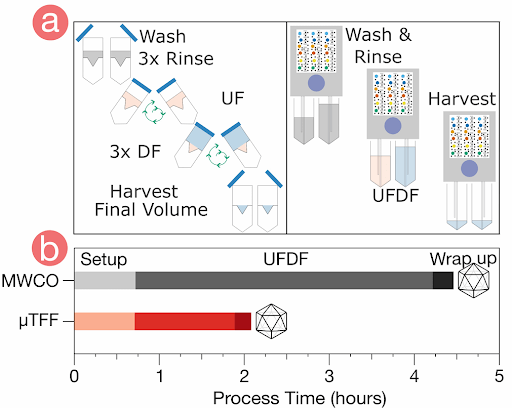
Compared to conventional methods, ultrafiltration addresses all the considerations for virus harvesting and final formulation. However, dead-end filtration (DEF), the commonly used form of ultrafiltration at lab scale, is limited by concentration gradient development at the membrane as the sample is not recirculated. This slows the filtration process and may result in material loss due to aggregation. Additionally, the only way to monitor the concentration progress or to mix the sample is by periodic interruption of the centrifugation, making it a hands-on method and adding further to the processing time. Lastly, the dead-end centrifugal devices lack scalability and can process only limited volumes.
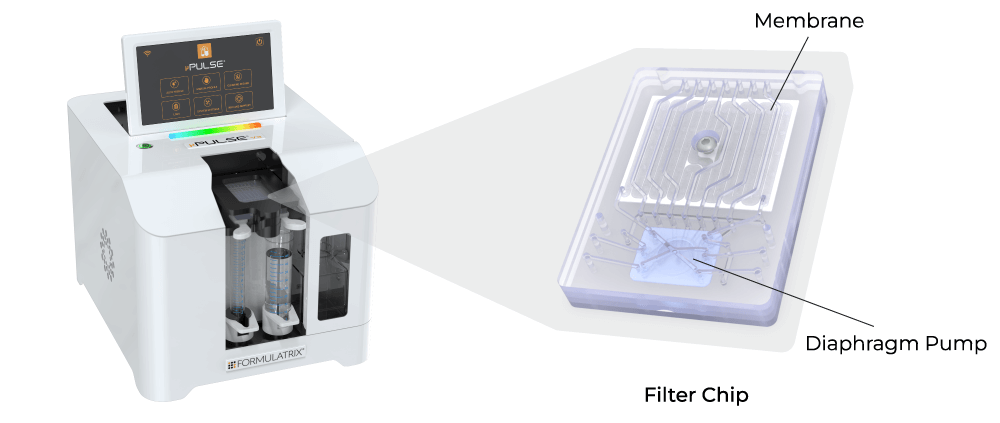
The limitations associated with DEF are effectively countered by tangential flow filtration (TFF), which features constant sample recirculation to avoid filter cake formation and ensuring high filtration rates. TFF also offers scalability and the advantage of simultaneous buffer exchange and sample concentration. However, traditional TFF systems have large footprints with hold-up volumes in tens to hundreds of milliliters, making them unsuitable for lab scale applications. To overcome this challenge, Formulatrix has developed the µPulse® - a miniaturized TFF system designed specifically for lab scale volumes.
Virus Processing Using the µPulse - TFF System
The Formulatrix µPulse is an automated and miniaturized TFF system explicitly designed for lab scale applications. It is well suited for processing AAVs, lentiviruses, bacteriophages, and VLPs. The entire fluid path is miniaturized on the filter chip by combining our patented microfluidic pumping technology with TFF. This has reduced the hold-up volume to just 0.65 mL, ensuring maximum hold-up recovery.
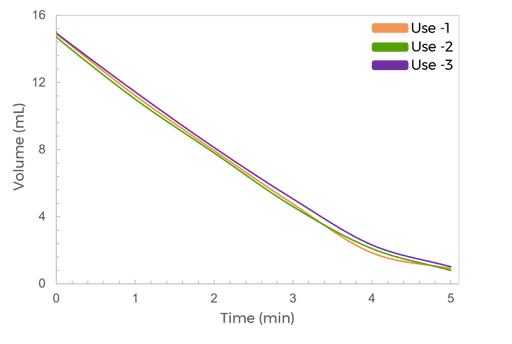
Its customizable settings, including adjustable pressures and pump parameters, provide a gentle yet highly efficient process for virus processing. The filter chips are available with modified polyethersulfone (mPES) and regenerated cellulose (RC) membranes that exhibit low fouling characteristics and are compatible with various sample types. Furthermore, it processes samples 4x faster than the dead-end centrifugal units and in a walk-away approach.
High-Throughput Virus Processing with the aµtoPulse - TFF System
The aµtoPulse® is a fully automated, high-throughput TFF system with the world’s lowest hold-up volume of just 250 µL. It processes up to 54 samples per run, with up to 4 samples in parallel. Designed for flexibility, it handles starting volumes between 0.5 mL to 100 mL and can concentrate samples down to 250 µL with ±25 µL precision. The system supports up to four external buffer inputs or on-deck conical tubes, enabling automated multi-buffer diafiltration.
The advanced chip design with dual pumps delivers permeate flow rates up to 1.7x faster than the µPulse while minimizing shear for sensitive viruses and virus-like particles (VLPs). The chips are available with mPES (5–300 kDa) and RC (5–100 kDa) membranes, ensuring compatibility with a wide range of samples.
Each station offers independent regulation and monitoring of transmembrane pressure (0–32 psi), giving users full control over process gentleness and efficiency. The intuitive, browser-based software enables remote protocol setup, monitoring, and control, with secure data management compliant with 21 CFR Part 11 for GMP environments.
Webinars
Explore the ease of use and cost-effectiveness of µPulse for processing VLPs and other macromolecules compared to dead-end units.
Discover a novel, automated, and walk away approach for sample concentration and buffer exchange at lab scale using the µPulse - a miniaturized TFF system.

Resources
Experience the efficiency, fast processing, and gentleness of the miniaturized µPulse - TFF system for concentration and buffer exchange of protein samples.
From Dyno Therapeutics
Streamline the processing of AAVs with high filtration rates and minimal hands-on time in a cost effective manner using the µPulse - TFF system.
FAQs
Is TFF suitable for viral vector concentration?
Yes, Tangential Flow Filtration (TFF) is a standard, scalable method for viral vector concentration, effectively used for adeno-associated viruses (AAV), lentiviruses, and other gene therapy vectors. Systems like the µPulse and aµtoPulse enable gentle processing with single-use, low-adsorption membranes that retain viral particles while removing impurities. Their precise control over pressure and cross-flow minimizes shear stress, preserving viral infectivity and integrity. The low hold-up volume and integrated product recovery maximize yield. This closed, automated approach is directly scalable from process development to GMP manufacturing, ensuring reproducibility and preventing cross-contamination.
Can TFF be used for simultaneous virus concentration and buffer exchange in a single run?
Yes, tangential Flow Filtration (TFF) is specifically designed to perform simultaneous virus concentration and buffer exchange—known as diafiltration—in a single, automated run. As the virus particles are retained and concentrated, the original buffer components, along with impurities, are progressively removed and replaced. Systems like the µPulse and aµtoPulse automate this integrated process with precise control over transmembrane pressure and cross-flow, ensuring high viral recovery (>90%) while maintaining infectivity and structural integrity. This approach significantly reduces processing time, minimizes sample handling, and lowers the risk of contamination, making it a scalable, efficient solution for viral vector processing in both research and GMP manufacturing.
How scalable is viral TFF from bench to GMP production?
Viral TFF processes are highly scalable from bench-scale systems like the µPulse and aµtoPulse to GMP production by maintaining critical parameters—transmembrane pressure, cross-flow velocity, membrane chemistry, and molecular weight cut-off—across scales. This ensures consistent product quality, infectivity, and yield during scale-up. Systems designed for GMP compliance offer closed processing, single-use flow paths, and data integrity features that streamline validation and prevent contamination. The low hold-up volumes of bench-scale systems maximize yield at small scale and provide reliable data for linear scale-up, enabling a seamless transition from process development to clinical manufacturing.