Sample concentration and buffer exchange are integral to any preparative scale protein production, whether for structural biology or general biochemistry needs. These steps are required to ensure specific chromatographic requirements in between the purification steps. Even after purification, the protein must be formulated in a specific buffer at appropriate concentrations to ensure its structural integrity and effectiveness for downstream applications. Similarly, buffer exchange may also be required for in vitro refolding of the solubilized inclusion bodies by controlled denaturant removal.
Given the importance of these steps, factors such as gentleness, time efficiency, scalability, and cost-effectiveness must be taken into account while selecting a method for protein processing. Tables 1 and 2 outline the typical methods employed for protein concentration and buffer exchange, respectively:
| Considerations |
Column Chromatography | Lyophilization | Evaporation | Absorption |
Ultrafiltration Dead-end TFF |
|---|---|---|---|---|---|
| Gentleness | ✔ | ✔ | ✔ ✔ | ||
| Scalability | ✔ | ✔ | ✔ | ✔ | ✔ |
| Time Efficiency | ✔ | ||||
| Cost Efficiency | ✔ | ✔ | ✔ ✔ | ||
| Automation | ✔ |
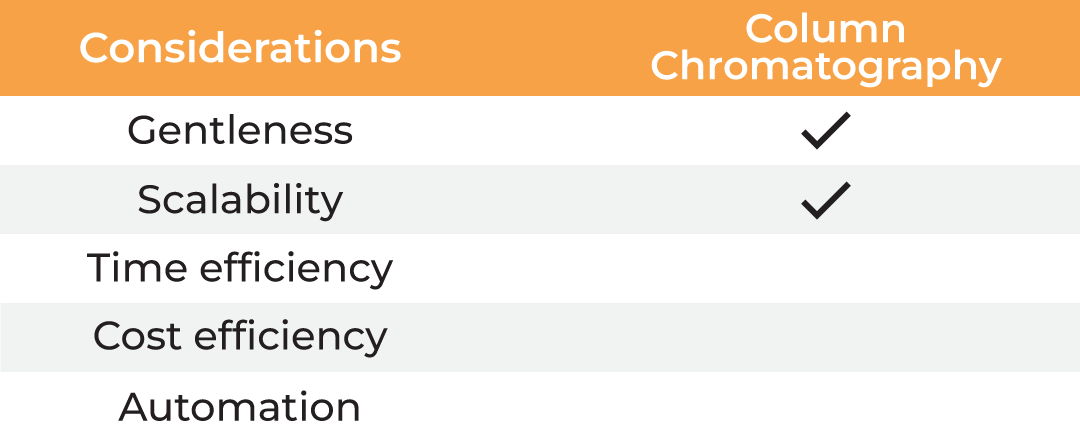
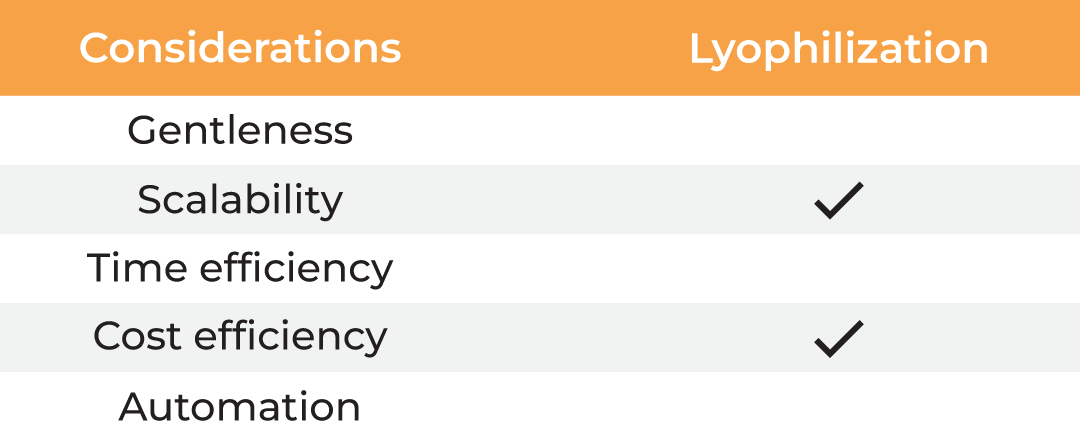
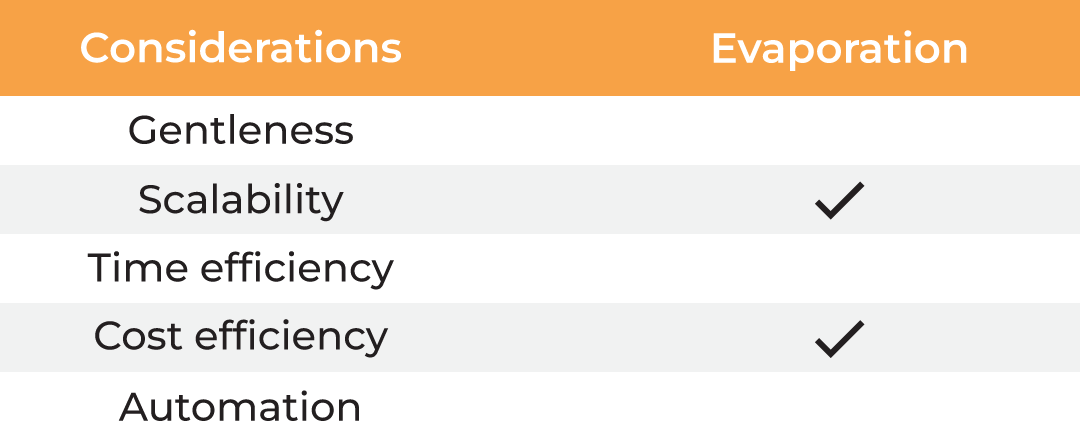
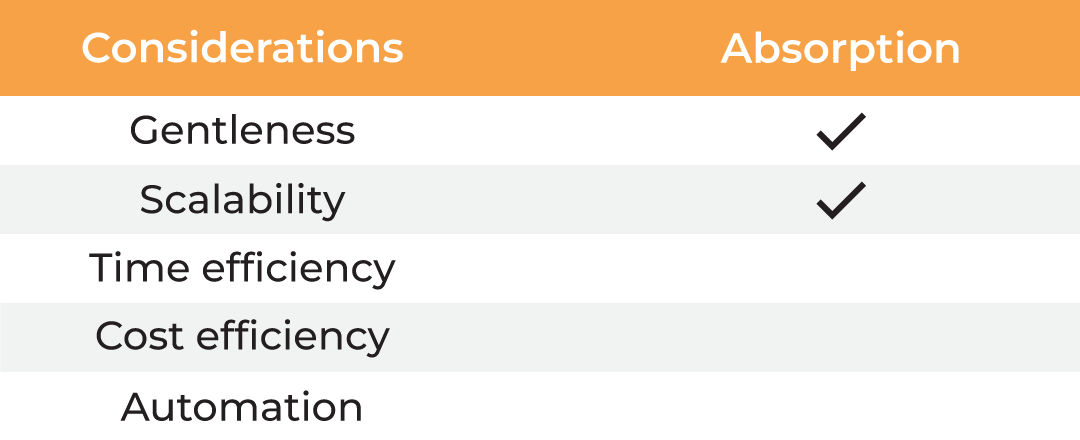
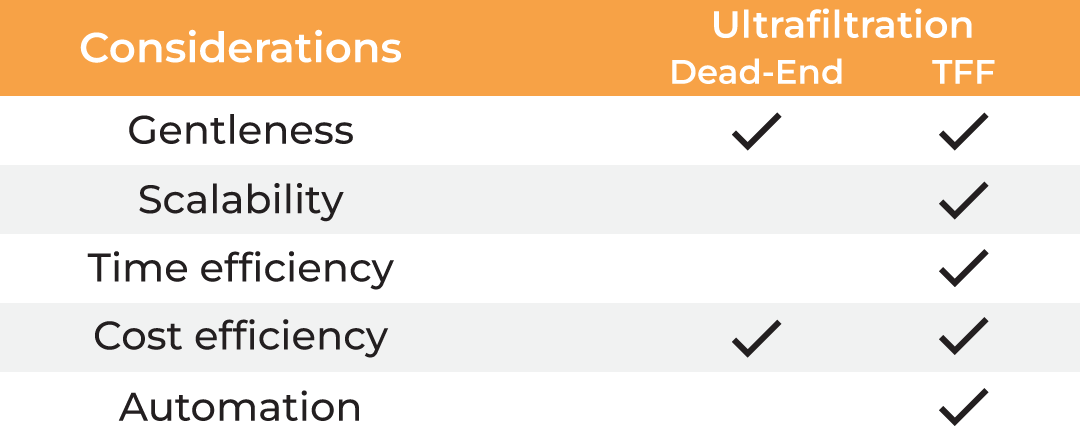
Table 1. Methods for protein concentration
Ultrafiltration addresses all the concerns for protein concentration as well as buffer exchange compared to conventional methods. However, dead-end ultrafiltration creates a concentration gradient at the membrane as the sample is not recirculated. This slows down the filtration process and may result in material loss due to aggregation. The only way to monitor the concentration progress or to mix the sample, is by periodic interruption of the centrifugation, making it a hands-on method and adding further to the processing time. Lastly, the dead-end centrifugal devices lack scalability and can process only limited volumes.
These concerns are effectively addressed by tangential flow filtration (TFF), an alternative form of ultrafiltration that involves constant sample recirculation, thus avoiding the filter cake formation and ensuring high filtration rates. TFF also offers scalability and advantage of simultaneous buffer exchange and sample concentration. However, traditional TFF systems have a large footprint with hold-up volumes in tens to hundreds of milliliters, making them unsuitable for lab scale applications.
| Considerations |
Column Chromatography | Dilution |
Equilibrium Dialysis |
Ultrafiltration Dead-end TFF |
|---|---|---|---|---|
| Gentleness | ✔ | ✔ | ✔ | ✔ ✔ |
| Scalability | ✔ | ✔ | ✔ | |
| Time Efficiency | ✔ | |||
| Cost Efficiency | ✔ ✔ | |||
| Automation | ✔ |
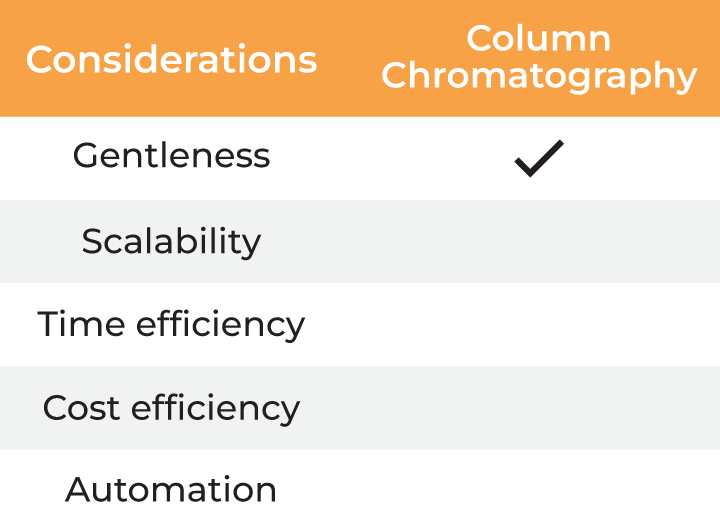
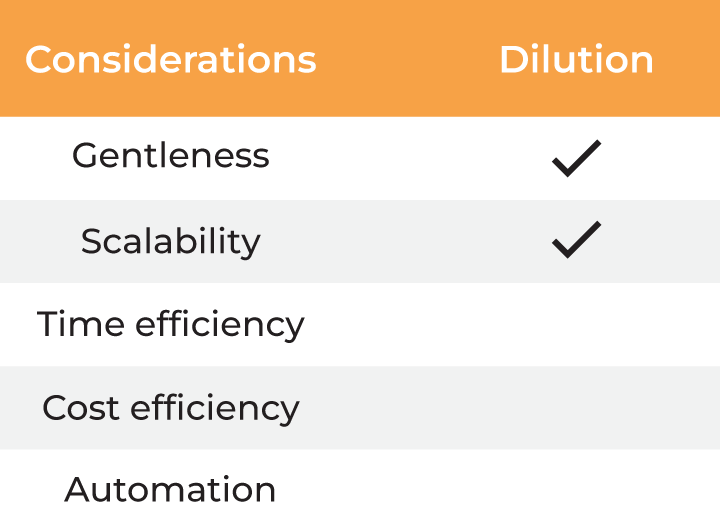
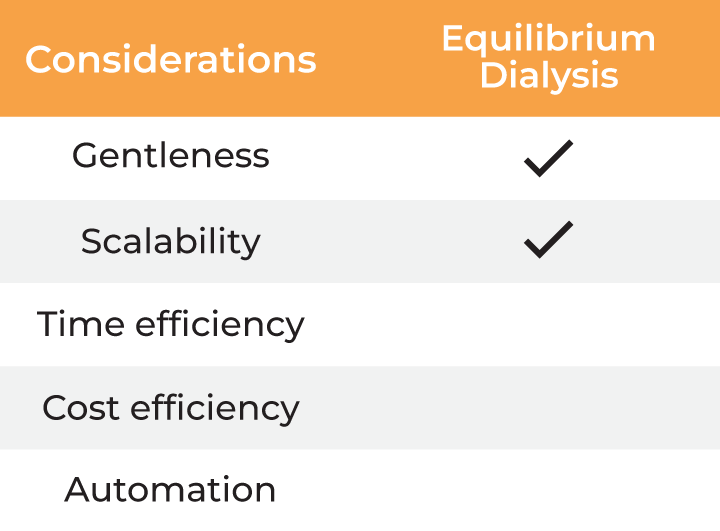
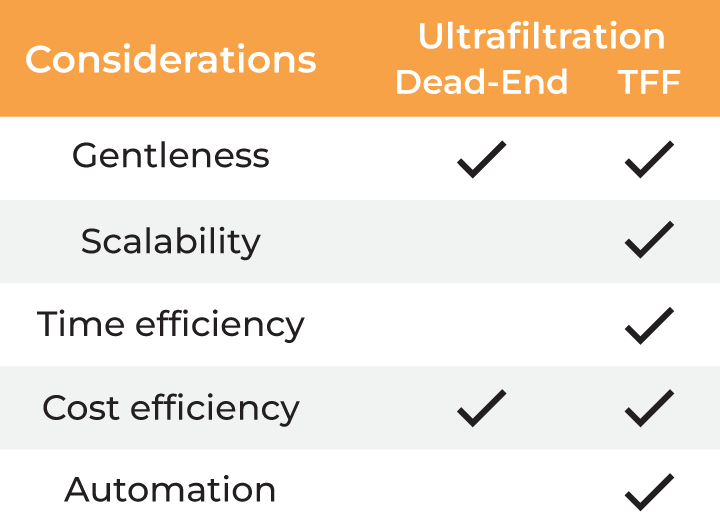
Table 2. Methods for buffer exchange
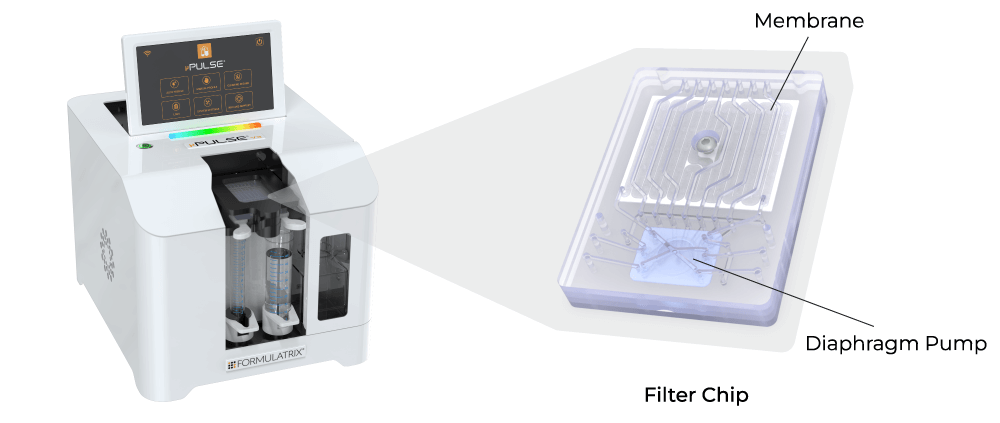
Protein Processing Using the µPulse - TFF System
The Formulatrix µPulse® is a fully automated and miniaturized TFF system, explicitly designed for sample concentration and diafiltration at lab scale. The entire fluid path is miniaturized on the filter chip by combining microfluidic pumping technology with TFF. This has reduced the hold-up volume to just 0.65 mL, ensuring maximum sample recovery.
The µPulse filter chips are currently available with modified polyethersulfone (mPES) and regenerated cellulose (RC) membranes that exhibit low fouling characteristics and are compatible with a variety of sample types. The µPulse offers customization for the operating pressures to make the process gentle, ensuring the structural integrity of proteins and other biomolecules. Furthermore, the µPulse processes samples 4x faster compared to dead-end centrifugal filters, and in a walk-away approach.
High-Throughput Protein Processing with the aµtoPulse - TFF System
The aµtoPulse® is a fully automated, high-throughput TFF system with the world’s lowest hold-up volume of just 250 µL. It processes up to 54 samples per run, with up to 4 samples in parallel. Designed for flexibility, it handles starting volumes between 0.5 mL to 100 mL and can concentrate samples down to 250 µL with ±25 µL precision. The system supports up to four external buffer inputs or on-deck conical tubes, enabling automated multi-buffer diafiltration.
The advanced chip design with dual pumps delivers permeate flow rates up to 1.7x faster than the µPulse while minimizing shear, particularly important for delicate proteins and complexes. The chips are available with mPES (5–300 kDa) and RC (5–100 kDa) membranes, ensuring compatibility with a wide range of samples.
Each station offers independent regulation and monitoring of transmembrane pressure (0–32 psi), giving users full control over process gentleness and efficiency. The intuitive, browser-based software enables remote protocol setup, monitoring, and control, with secure data management compliant with 21 CFR Part 11 for GMP environments.
Webinars
Learn a fast, gentle, and automated approach for harvesting extracellular VLPs as well as refolding, and formulating various proteins using the µPulse.
Experience the single-step and scalable purification of ADCs and other macromolecules modified with small molecules using the µPulse.
Application Notes
Uncover the ease of use, scalability, and reusability of µPulse for lab scale formulation of recombinant L-asparaginase and other biomolecules.

Learn about the time efficiency, cost effectiveness and gentleness of µPulse for refolding denatured proteins compared to equilibrium dialysis.
Publications
FAQs
How does tangential flow filtration (TFF) compare to centrifugation for protein purification?
TFF is superior to centrifugation for most protein purification workflows due to its higher efficiency, scalability, and automation, while centrifugation remains useful for initial crude clarification. TFF continuously recirculates the sample across the membrane, maintaining stable flux and uniform concentration. This results in higher recovery, better reproducibility, and improved protein stability, especially for shear- or aggregation-sensitive proteins. In addition, automated TFF systems such as the µPulse and aµtoPulse enable controlled transmembrane pressure (TMP), faster processing, and integrated buffer exchange in a single run—saving time and reducing hands-on handling compared to repeated centrifugation steps.
How do I integrate TFF into an existing chromatography workflow?
Tangential flow filtration (TFF) can be integrated into an existing chromatography workflow as a complementary step for concentration and buffer exchange. Typically, after initial capture or intermediate purification using chromatography (e.g., Protein A, ion exchange, or affinity columns), the eluate can be processed via TFF to concentrate the product and exchange it into the desired formulation buffer before polishing or final formulation steps. Automated systems like µPulse and aµtoPulse simplify this integration by providing controlled transmembrane pressure, low hold-up volumes, and reproducible recovery, ensuring minimal product loss and consistent quality. Incorporating TFF in this way reduces hands-on time, increases throughput, and allows seamless scale-up from lab to pilot or GMP production.
What are the typical processing times for protein concentration and buffer exchange using TFF compared to dialysis?
TFF is significantly faster than dialysis for protein buffer exchange. While traditional dialysis can take several hours to overnight due to passive diffusion, automated TFF systems like µPulse and aµtoPulse can process small- to medium-volume protein samples in minutes to under an hour, depending on sample volume and membrane MWCO. This speed is achieved through active tangential flow, controlled transmembrane pressure, and continuous permeate removal, which not only shortens processing time but also maintains high recovery and protein integrity.