Nanoparticles are specialized structures typically ranging in size from 1 to 100 nm. The term may also refer to larger particles (up to 500 nm), or fibers and tubes <100 nm in at least two dimensions. Owing to their nanoscale size and high surface area-to-volume ratio, nanoparticles are widely used in diagnostics, drug delivery systems, and therapeutics. Some common examples of nanoparticles include lipid nanoparticles (LNPs), liposomes, endogenous nanoparticles (exosomes), metal nanoparticles (MNPs), and polymeric nanoparticles.
A typical production workflow for nanoparticles involves four key steps: synthesis, harvesting, purification, and final formulation. The nanoparticles are synthesized using physical, chemical, and biological methods, each providing unique advantages for controlling particle size, shape, and composition tailored to specific applications.
After synthesis, nanoparticles are harvested using methods such as centrifugation, evaporation, ultrafiltration, or precipitation followed by purification. Keeping in view the stability, structural integrity, and effectiveness for downstream applications, nanoparticles are formulated using techniques such as dialysis, chromatography, or ultrafiltration. Therefore, factors like time efficiency, scalability, yield, and gentleness are crucial when choosing methods for harvesting and final formulation.
Production workflow of nanoparticles
Tables 1 and 2 present various techniques for harvesting and final formulation of nanoparticles, with ultrafiltration emerging as the most suitable method. However, despite being a common method, ultrafiltration using dead-end devices (called dead-end filtration or DEF) is limited by low filtration rates, scalability challenges, frequent sample loss, and manual interventions.
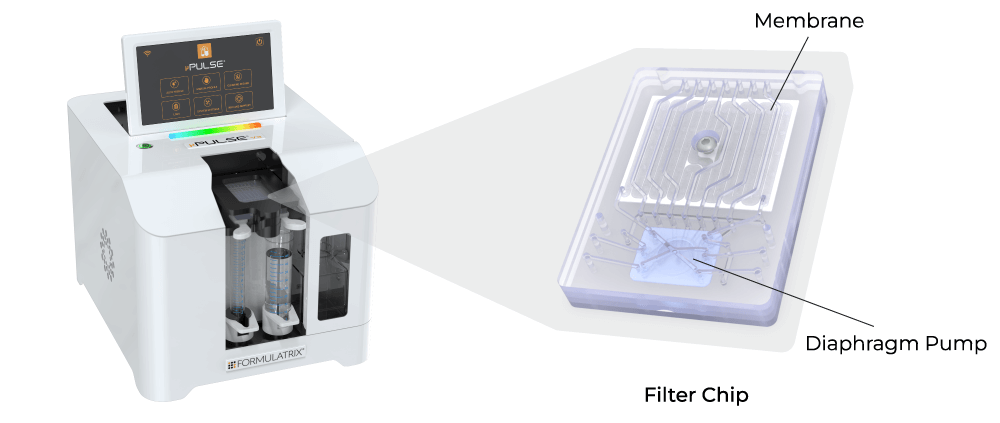
In contrast, ultrafiltration in the form of tangential flow filtration (TFF) offers higher filtration rates and scalability. However, traditional TFF systems are typically large and have high hold-up volumes, making them unsuitable for lab scale applications. To overcome these limitations, Formulatrix has developed the µPulse® - an automated and miniaturized TFF system designed specifically for sample concentration and buffer exchange at the lab scale.
| Considerations | Precipitation | Centrifugation | Evaporation |
Ultrafiltration Dead-End TFF |
|---|---|---|---|---|
| Gentleness | Moderate | Moderate | Low | Moderate High |
| Scalability | Moderate | Moderate | Moderate | Moderate High |
| Time Efficiency | Low | Low | Low | Low High |
| Cost Efficiency | Low | High | Moderate | Moderate High |
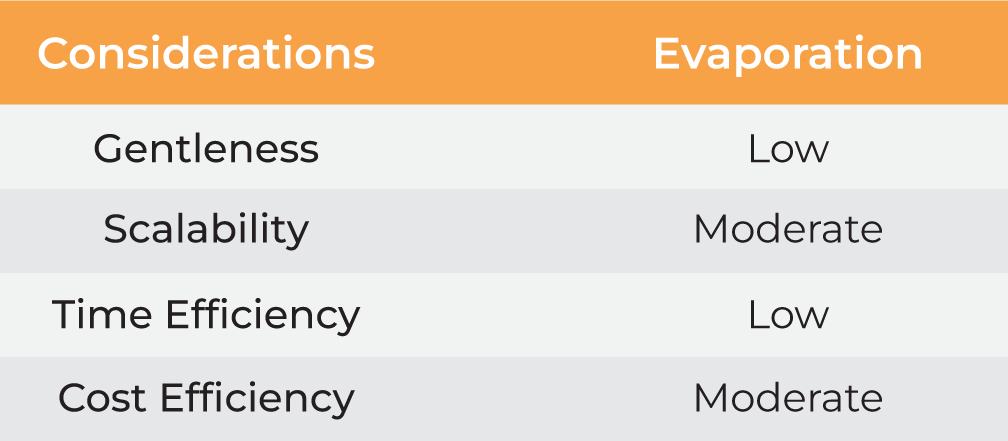
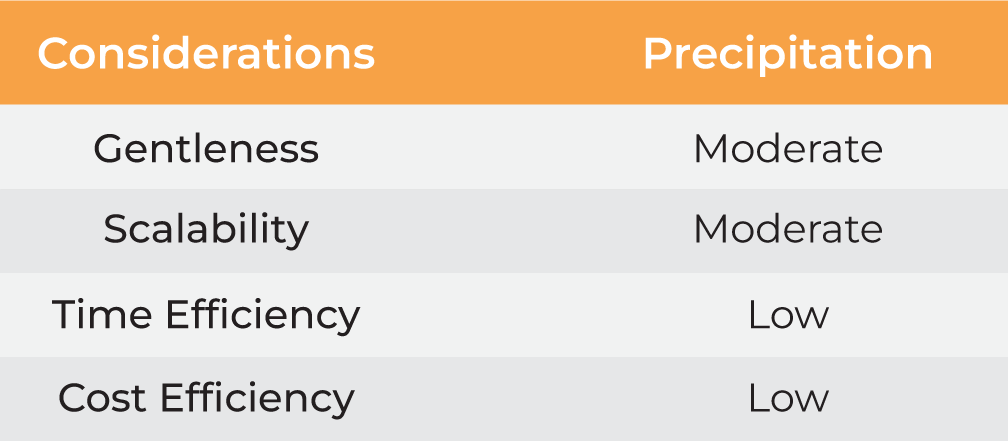
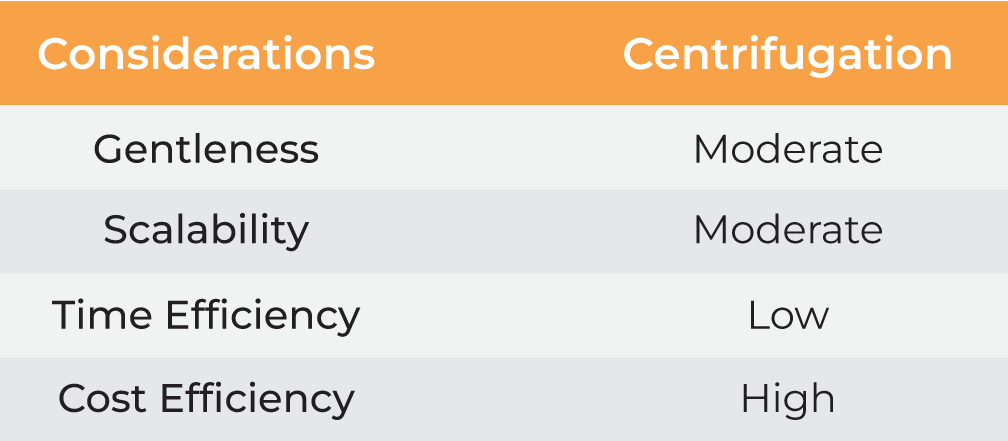
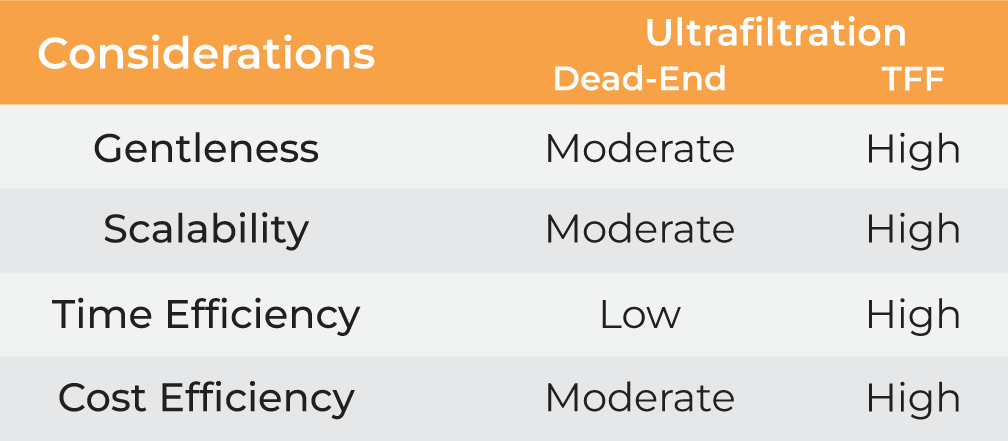
Table 1. Methods for nanoparticle harvesting
| Considerations | Dialysis | Chromatography |
Ultrafiltration Dead-End TFF |
|---|---|---|---|
| Gentleness | High | High | Moderate High |
| Scalability | Moderate | Moderate | Moderate High |
| Time Efficiency | Low | Low | Low High |
| Cost Efficiency | Moderate | Moderate | Moderate High |
Table 2. Methods for nanoparticle formulation
Nanoparticle Processing Using the µPulse - TFF System
The µPulse is an automated and miniaturized TFF system, explicitly designed for lab scale applications. It is well suited for the purification of a variety of nanoparticles including LNPs, liposomes, exosomes, MNPs, and polymeric nanoparticles.
The entire fluid path is miniaturized on the filter chip by combining microfluidic pumping technology with TFF. This has reduced the hold-up volume to just 0.65 mL, ensuring maximum hold-up recovery. The filter chips are available with modified polyethersulfone (mPES) and regenerated cellulose (RC) membranes that exhibit low fouling characteristics and are compatible with a variety of sample types.
The µPulse offers customizable parameters such as operating pressures and pump settings to optimize the processing of a variety of nanoparticles. Furthermore, it processes samples 4x faster compared to the dead-end centrifugal units, and in a walk-away approach.
Webinars
Discover how the silver nanoparticles are processed in a fast, single-step, and walk-away approach with the µPulse.
Learn a rapid, gentle, and automated approach for exosome harvesting and final formulation using the µPulse.
Application Note
Discover how the µPulse - TFF system streamlines exosome harvesting with gentle, fast processing that preserves particle integrity, enhances functionality, reduces manual steps, and accelerates lab-scale workflows.
FAQs
Which TFF membranes are best for extracellular vesicle purification?
For extracellular vesicle (EV) purification, mPES TFF membranes with higher MWCOs (typically 100–300 kDa or above) are best suited because they provide high permeate flux and low fouling while effectively retaining vesicles such as exosomes and microvesicles. In automated systems like the µPulse and the aµtoPulse, controlled transmembrane pressure and gentle tangential flow preserve EV integrity while allowing smaller proteins and impurities to pass through. Selecting the right MWCO and membrane material improves EV recovery, shortens processing time, and delivers higher purity compared to centrifugation-based methods.
Can a lab-scale TFF system be adapted for exosome isolation?
Yes, a lab-scale TFF system can be effectively adapted for exosome isolation. Systems like the µPulse and the aµtoPulse enable gentle, low-shear processing using high-MWCO membranes (typically ≥100–300 kDa) that retain exosomes while allowing smaller contaminants to pass through. The controlled transmembrane pressure, low hold-up volumes, and automated diafiltration improve exosome recovery, purity, and reproducibility compared to ultracentrifugation, making lab-scale TFF a practical and scalable approach for exosome processing.
What strategies can be used to maximize recovery of delicate nanoparticles such as exosomes or LNPs?
To maximize recovery of delicate nanoparticles such as exosomes or LNPs, it is important to use low-shear tangential flow with carefully controlled transmembrane pressure (TMP) to prevent aggregation and structural damage. Selecting the appropriate MWCO membrane (e.g. 300 kDa) ensures nanoparticles are retained while smaller impurities are removed. Systems with low hold-up volumes, such as the µPulse and the aµtoPulse, minimize dead zones and sample loss, while gentle buffer exchange conditions—including optimal pH, ionic strength, and temperature—preserve particle integrity. Additionally, using single-use chips or validated cleaning protocols prevents cross-contamination and supports reproducible recovery, resulting in high-yield, consistent nanoparticle concentration and diafiltration workflows.


