Automate Your Lipidic Cubic Phase Workflow
Lipidic cubic phase (LCP) is an excellent medium for the crystallization of membrane proteins and is gaining popularity in the top pharmaceutical labs worldwide. Formulatrix offers an entire suite of products that automate each phase of the LCP workflow: dispensing, screening crystallization conditions, and protein crystal imaging and identification.
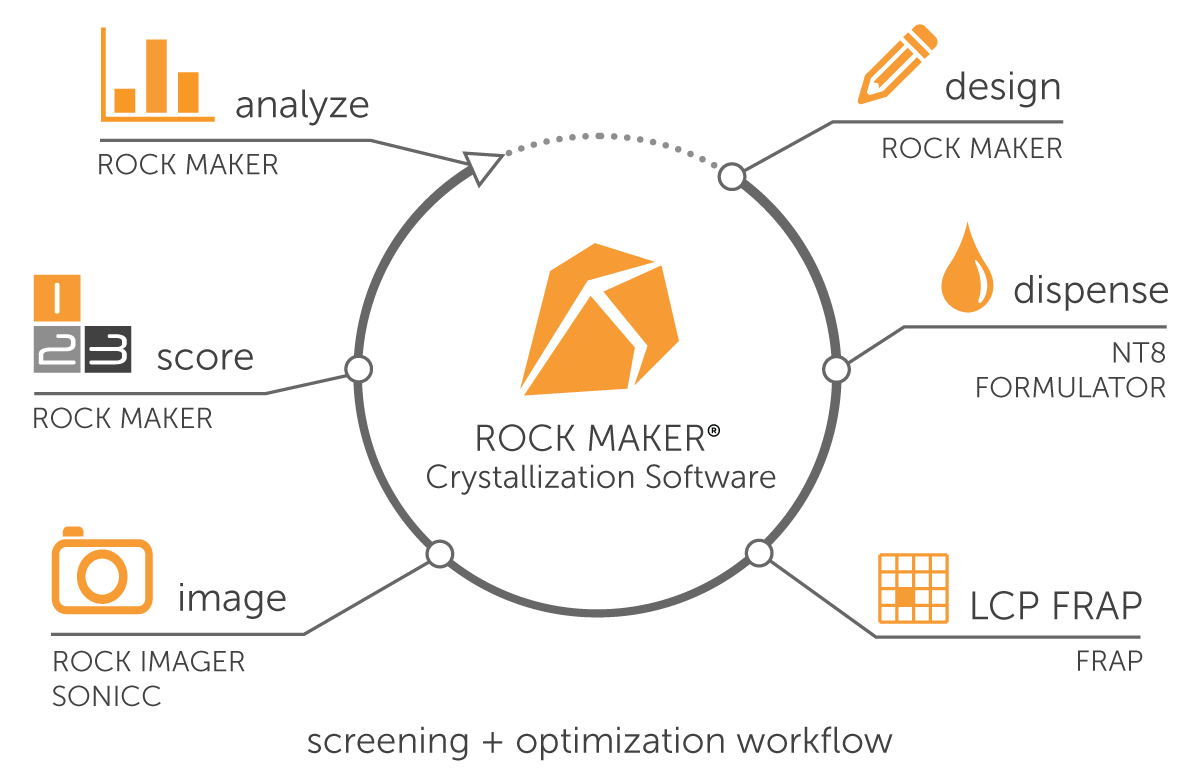
Manage the entire protein crystallization experimentation process, from design and dispense to image viewing and analysis all from one software, Rock Maker.
Rock Maker is a Laboratory Information Management System (LIMS) that is there every step of the way, with powerful yet easy-to-use tools that help you quickly get the results you need.
Rock Maker integrates with:
Rock Imager® - Crystallization Imagers
Reliable Drop Setting: Reduce Costs + Increase Reproducibility
- Set up hanging drop, sitting drop, microbatch, in meso Lipidic Cubic Phase (LCP) drops, additive, and seeding experiments
- 8-tip head aspirates and dispenses drops from 10 nL to 1.5 μL
- Reusable tips give you the option to dispose of tips after each dispense or reuse them to reduce costs and environmental waste
- Proportionally-Controlled Active Humidification minimizes sample evaporation and increases experiment reproducibility

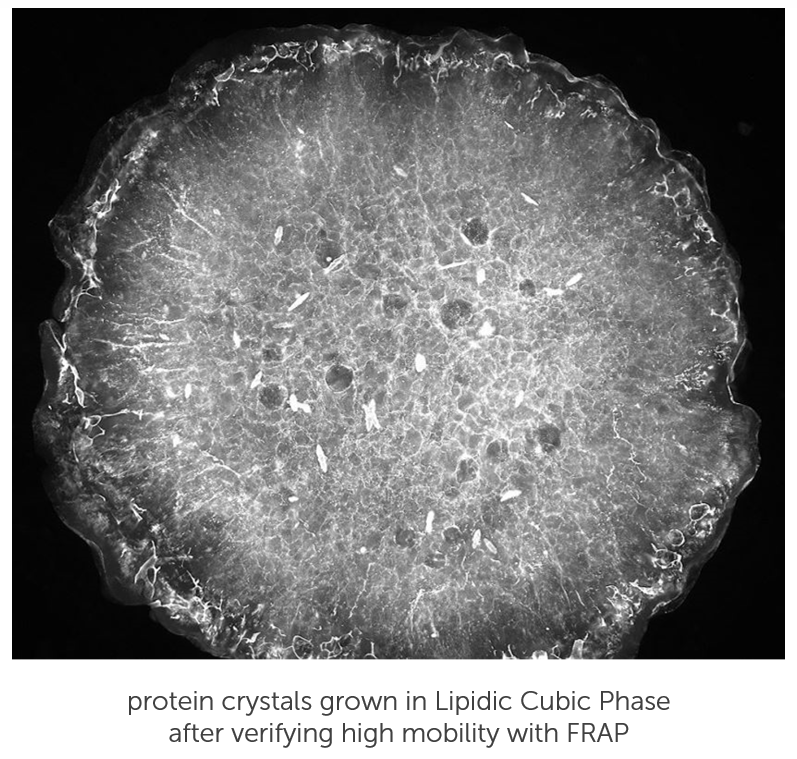
Find the Optimal Conditions for LCP Crystal Growth, Without Waiting on Your Crystals.
Prior to setting up crystallization experiments, you can quickly screen Lipidic Cubic Phase (LCP) conditions using Fluorescence Recovery After Photobleaching (FRAP). FRAP is an optical technique that assesses how easily protein is moving about in an LCP drop. If protein is aggregated, or the LCP structure is collapsed, the protein will not diffuse and, therefore, never crystallize. FRAP is a great tool to accelerate crystallization methods and rule out suboptimal conditions without having to wait on the crystals actually growing.
The automated FRAP imaging system was designed specifically for performing the FRAP assay on 96-well LCP crystallization setups. The entire system is automated and user-friendly, requiring a few clicks of a button to initiate a complete analysis of your plate. All of the results and analyses are integrated into Formulatrix's protein crystallography specific software lab management software (Rock Maker and Rock Imager).
FRAP was developed in collaboration with Vadim Cherezov (TSRI) and the JCIMPT center led by Ray Stevens and supported by the NIH Common Fund in Structural Biology.
Crystal Clear Imaging. Unparalleled Speed.
The most trusted imager in protein crystallization - used by the top 10 pharmaceutical companies and many renowned academic centers
Screen conditions with various imaging methods - Visible Light, UV fluorescence, UV absorption, Multi-Fluorescence Imaging, Fluorescence Recovery After Photobleaching (FRAP), and Second Order Nonlinear Imaging of Chiral Crystals (SONICC)
Various models to fit your budget and workflow - plate capacities from a single plate up to 970 SBS plates or 1500 LCP plates
Seamless integration with ROCK MAKER or your current crystallization software via Rock Imager API
Temperature regulation options available for precise temperature control from 30°C down to 4°C
Multiple plate options available - SBS, Linbro, Nextal, Terasaki/HLA, and Lipid Cubic Phase (LCP) plate
Remove the Guesswork.
SONICC Finds Crystals that Other Technologies Cannot.
The ultimate imaging technology in visualizing protein crystals, Second Order Nonlinear Imaging of Chiral Crystals (SONICC) definitively finds crystals buried in precipitate or submicron crystals naked to the eye. Two powerful technologies, Second Harmonic Generation (SHG) and Ultraviolet Two-Photon Excited Fluorescence (UV-TPEF), are combined in a completely automated imager to quickly image your high throughput crystallization plates and positively identify protein crystals.
Crystals appear white against a stark black background, helping you to identify crystals even in murky environments. SONICC can detect extremely thin crystals, microcrystals <1 μm, and crystals obscured in birefringent LCP.
Major pharmaceutical and academic research labs worldwide find SONICC to be extremely successful, cost effective, and efficient.
FAQs
What is lipidic cubic phase (LCP) protein crystallization, and how does it work?
Lipidic Cubic Phase (LCP) protein crystallization is a powerful technique designed for growing high-quality crystals of membrane proteins. It works by mixing specific lipids, most commonly monoolein, with an aqueous protein solution, forming a highly organized, bicontinuous lipid bilayer known as the cubic phase. This structure closely mimics the natural environment of membrane proteins, helping them maintain their native conformation and stability. Within this lipid matrix, proteins can diffuse slowly, allowing ordered crystal growth under near-physiological conditions. As a result, LCP provides an ideal platform for obtaining well-diffracting crystals of membrane proteins that are often challenging to crystallize using traditional aqueous methods.
Why is the lipidic cubic phase method widely used for membrane protein crystallization?
The lipidic cubic phase (LCP) method is widely used for membrane protein crystallization because it provides a near-native environment that closely mimics the natural lipid bilayer of cell membranes. This environment helps membrane proteins maintain their native conformation, structural integrity, and biological activity. Moreover, LCP allows proteins to remain fully mobile within the lipid bilayer, promoting the formation of well-ordered crystals suitable for high-resolution structural studies.
What are the common challenges and limitations in LCP protein crystallization?
Crystallizing membrane proteins in lipidic cubic phase (LCP) presents several practical challenges. The cubic phase itself is highly viscous—similar to thick toothpaste—making it difficult to handle and dispense with precision. Early methods required multiple centrifugations in small glass tubes, making mixing and crystal harvesting tedious, inefficient, and prone to sample loss. Specialized tools, such as coupled-syringe mixers, are now used to combine microliter volumes of lipid and protein solution, but they still require technical skill and careful handling to minimize waste. Detecting small crystals, often only a few micrometers in size, can also be challenging without high-quality optics or cross-polarized light. Furthermore, LCP crystallization experiments can take several months to complete, requiring long-term stability and consistent environmental conditions throughout the incubation period.