Ultrafiltration (UF) is a pressure-driven filtration process that utilizes a semipermeable membrane with pore sizes ranging from 0.01 to 0.1 µm. The pore size defines the nominal molecular weight cutoff (MWCO) of a membrane, specifying the minimum molecular weight of the particle effectively retained by a membrane.
UF is effectively used for sample concentration, diafiltration (buffer exchange, desalting), and size fractionation. These processes are integral to any preparative scale purification workflows involving macromolecules to maintain them at appropriate concentrations and in a suitable buffer for optimal functioning.
Modes of Ultrafiltration
There are two fundamental modes of ultrafiltration:
Dead-End Filtration
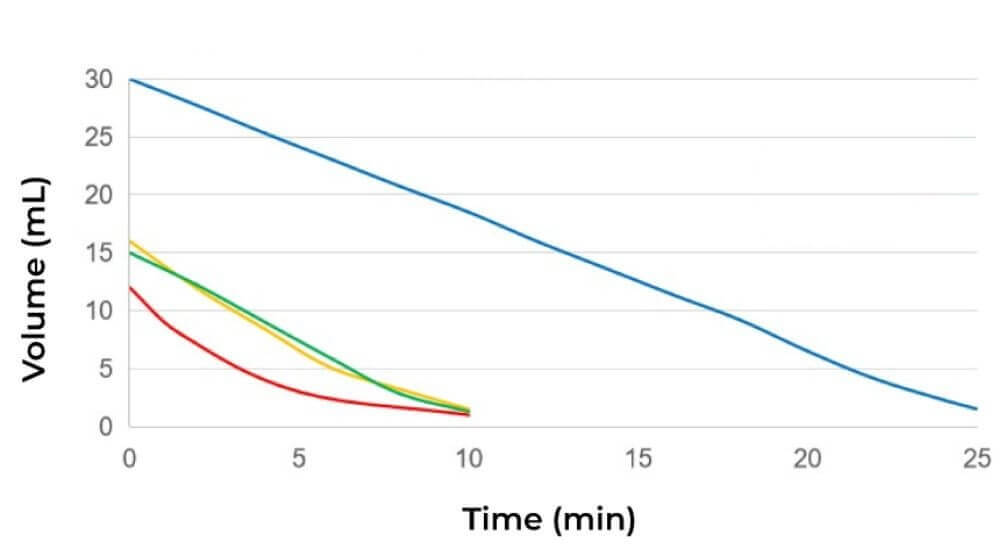
In dead-end filtration (DEF), the feed flows perpendicular to the membrane surface and the pressure for filtration is generated by centrifugal force. The dead-end devices only have a feed and permeate stream, while the retentate is not recirculated. This allows the entire feed stream to be pushed through the membrane and the small molecules are collected at the permeate side.
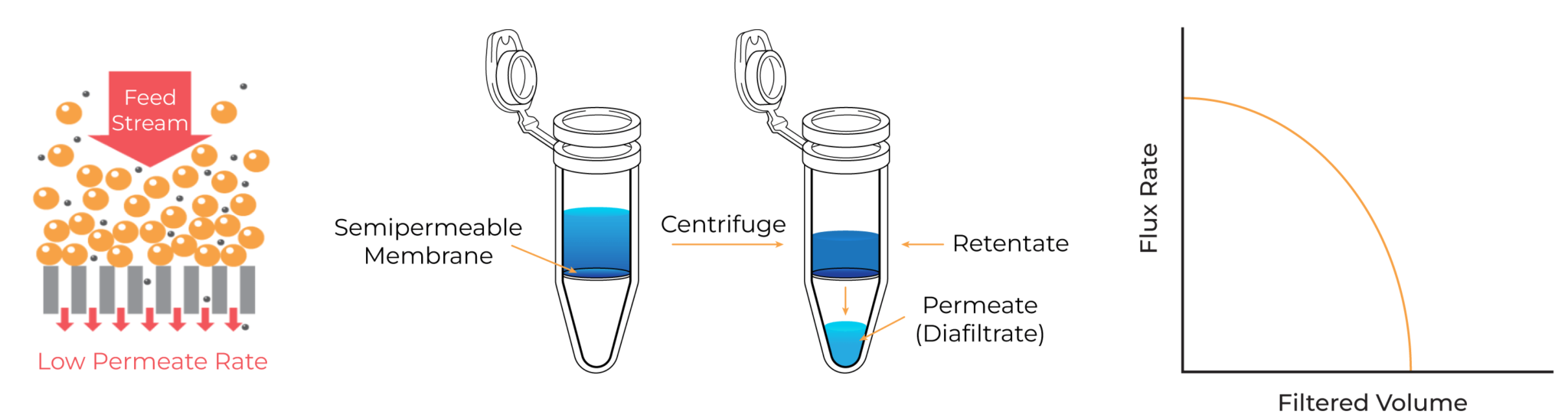
Dead-End Filtration
The simplicity of this mode makes it a popular choice for small-scale operations and laboratory studies. However, ultrafiltration using dead-end devices is limited by low filtration rates due to filter cake formation and lack of scalability. Because it is a hands-on method, it is often accompanied by sample loss. Additionally, these devices/membranes can be difficult to clean due to the extensive plugging of the narrow fibers/channels.
Tangential-Flow Filtration
The tangential-flow filtration (TFF) has the crossflow geometry in which the feed stream is pumped parallel to the membrane surface and perpendicular to the filtrate. Unlike DEF, TFF requires three distinct process streams: the feed, the permeate, and the retentate. In order to obtain high rates of mass transfer, it is necessary to have high tangential velocity and/or turbulence in the immediate vicinity of the membrane. This tangential velocity and turbulence help to prevent filter cake formation on the membrane and eventually avoid the decline in flux rate.
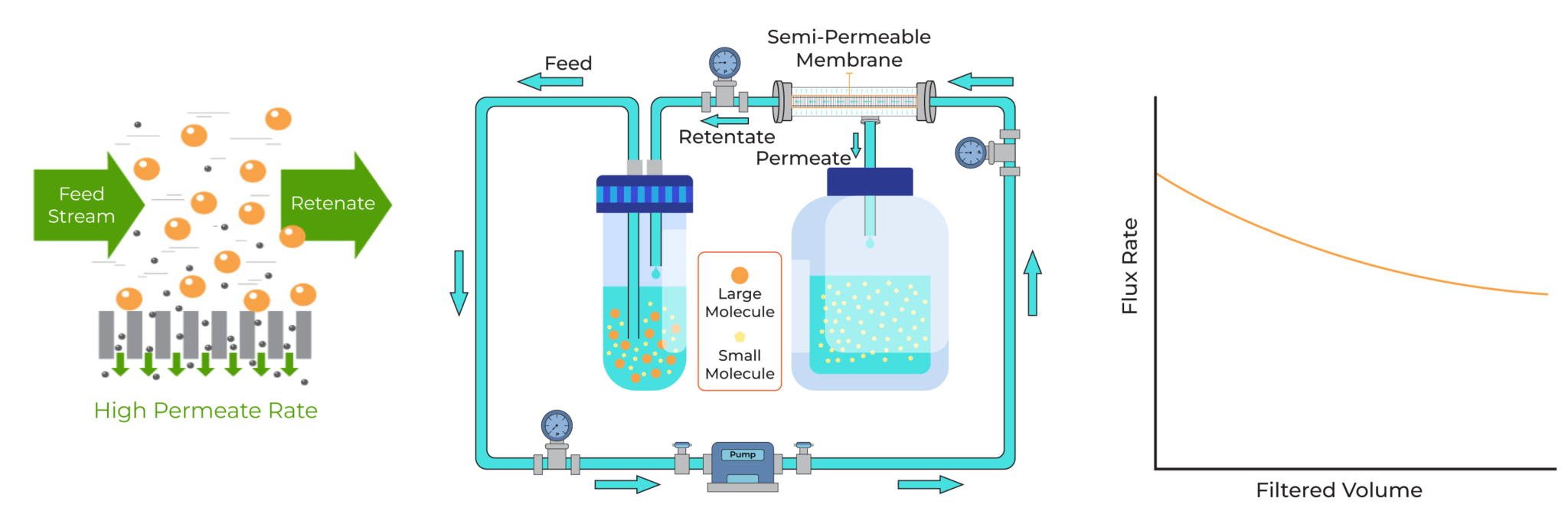
Tangential-Flow Filtration
The basic components of a conventional TFF include a reservoir, a pump, the membrane, the tubes that connect these components, and the pressure gauges. The pressure gauges, feed pressure, and return pressure present before and after the membrane, respectively, regulate the filtration rate by creating transmembrane pressure (TMP), which drives the small molecules through the membrane pores.
TFF Modules
Hollow Fiber
This module utilizes numerous hollow, narrow-diameter (0.1-2 mm) membrane tubes in the form of bundles. Feed is pumped inside the tubes, and the small molecules permeate through the walls of the tubes. This open path minimizes shear stress due to moderate cross-flow rates making it ideal for the processing of shear-sensitive products. However, hollow fibers have low efficiency as they require high pumping capacity to achieve high flux rates.
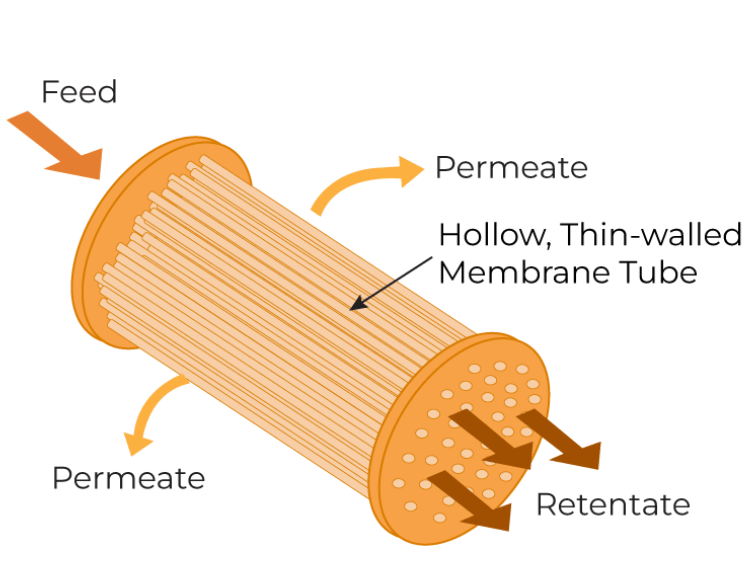
Hollow Fiber
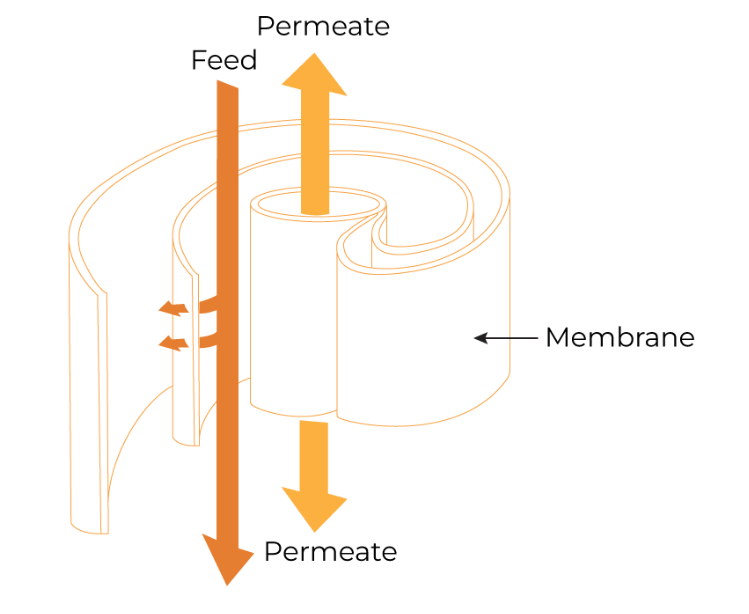
Spiral Wound
Spiral Wound
Spiral wound modules consist of alternating membrane and separator layers wrapped around a central core. Feed is pumped axially through the cartridge, while filtrate permeates the membrane and radially spirals towards the core. Separator screens enhance turbulence, boosting efficiency as compared to hollow fibers. The main drawback to spiral wound modules is that they are not linearly scalable because either the feed flow path length (cartridge length) or the filtrate flow path length (cartridge width) must be changed within scales. Despite this, their low cost and high membrane surface area make them excellent choices for large-scale food and beverage applications.
Flat Plate
Flat plate membrane module consists of single or stacked layers of membranes, potentially interspersed with separator screens, enclosed in a sealed unit. This module offers high membrane packing density, resulting in a significant membrane surface area per unit footprint. The feed solution, applied to one side of the membrane, traverses through the channels, and the permeate is collected from the other side. The flat plate module is a better option for shear-sensitive feeds and offers high flow rates as well as ease of cleaning.
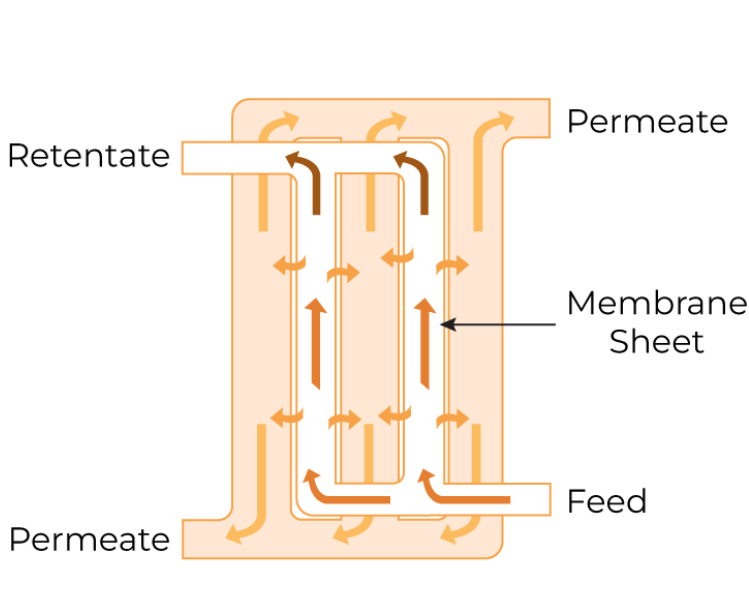
Flat Plate
Overall, the TFF effectively addresses the limitations associated with DEF such as lack of scalability, low filtration rate, aggregation and sample loss. However, the conventional TFF systems are bulky and have longer fluid paths, resulting in high hold-up volumes, making them unsuitable for lab scale applications. Therefore, an automated TFF system with a small footprint and minimal hold-up volume is well suited for laboratory research and experimentation.
The µPulse: An Automated and Miniaturized TFF System
The µPulse® is an automated and miniaturized TFF system designed explicitly for lab scale applications. The entire fluid path is miniaturized on a filter chip that has been designed by combining the TFF with microfluidic pumping technology. This has drastically reduced the hold-up volume to 0.65 mL, making it well suited for lab scale applications. The filter chips can be cleaned in place for re-use up to 300 mL of permeate and are available with modified polyethersulfone (mPES) and regenerated cellulose (RC) membranes in a range of MWCOs (5 - 300 kDa). The table below provides information about the selection of MWCO membrane to process various biomolecules. Compared to dead-end centrifugal units, the µPulse offers up to 4x higher filtration rates. While the dead-end centrifugal units require manual intervention, the weight-based volume sensing in µPulse ensures effective control on final volume, enabling single step and walk-away sample processing.
The aµtoPulse: High-throughput Sample Concentration - Diafiltration
The aµtoPulse® is a fully automated, high-throughput TFF system with the world’s lowest hold-up volume of just 250 µL. It processes up to 54 samples per run, with up to 4 samples in parallel. Designed for flexibility, it handles starting volumes between 0.5 mL to 100 mL and can concentrate samples down to 250 µL with ±25 µL precision. The system supports up to four external buffer inputs or on-deck conical tubes, enabling automated multi-buffer diafiltration.
The advanced chip design with dual pumps delivers permeate flow rates up to 1.7x faster than the µPulse while minimizing shear, particularly important for delicate samples. The chips are available with mPES (5–300 kDa) and RC (5–100 kDa) membranes, ensuring compatibility with a wide range of samples.
Each station offers independent regulation and monitoring of transmembrane pressure (0–32 psi), giving users full control over process gentleness and efficiency. The intuitive, browser-based software enables remote protocol setup, monitoring, and control, with secure data management compliant with 21 CFR Part 11 for GMP environments.
| Membrane MWCO (kDa) | Molecular/Particle Size (nm) | Protein (kDa) | Double Stranded Nucleic Acid (bp) | Single Stranded Nucleic Acid (bs) |
|---|---|---|---|---|
| 5 | 3-5 | 15-30 | 25-50 | 50-95 |
| 10 | 5-9 | 30-90 | 50-145 | 90-285 |
| 30 | 9-15 | 90-180 | 145-285 | 285-570 |
| 50 | 15-30 | 150-300 | 240-475 | 475-950 |
| 100 | 30-90 | 300-900 | 475-1450 | 950-2900 |
| 300 | 90-200 | 900-1800 | 1450-2900 | 2900-5700 |
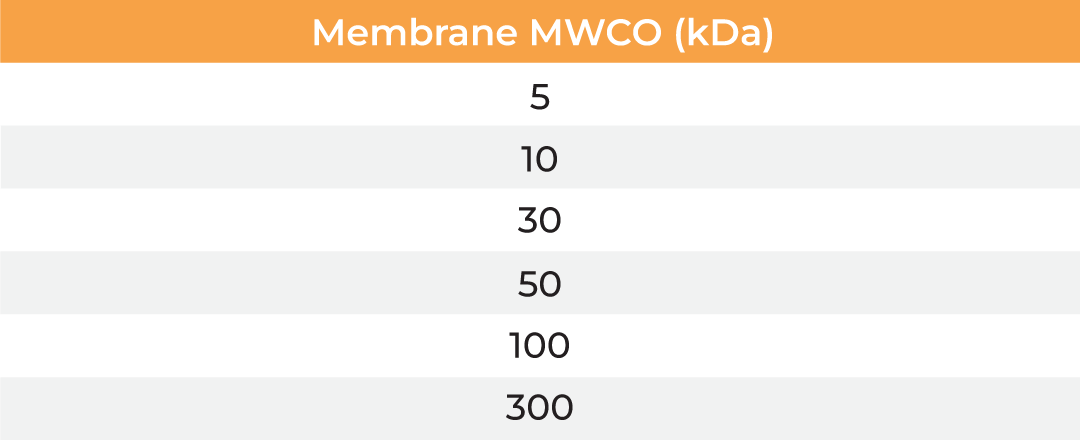
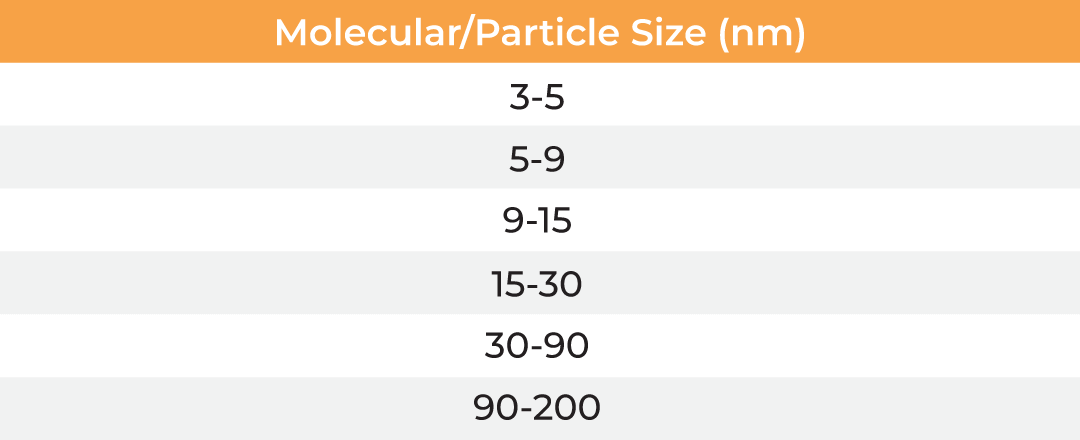
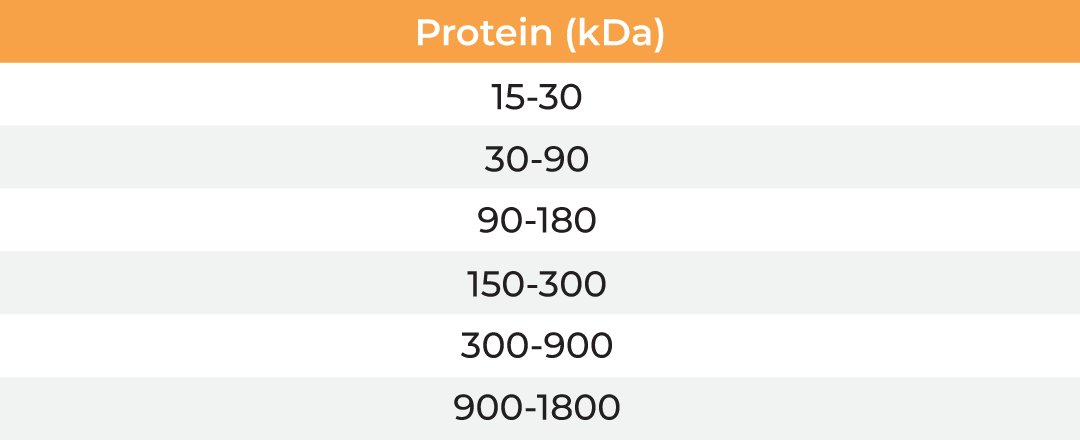
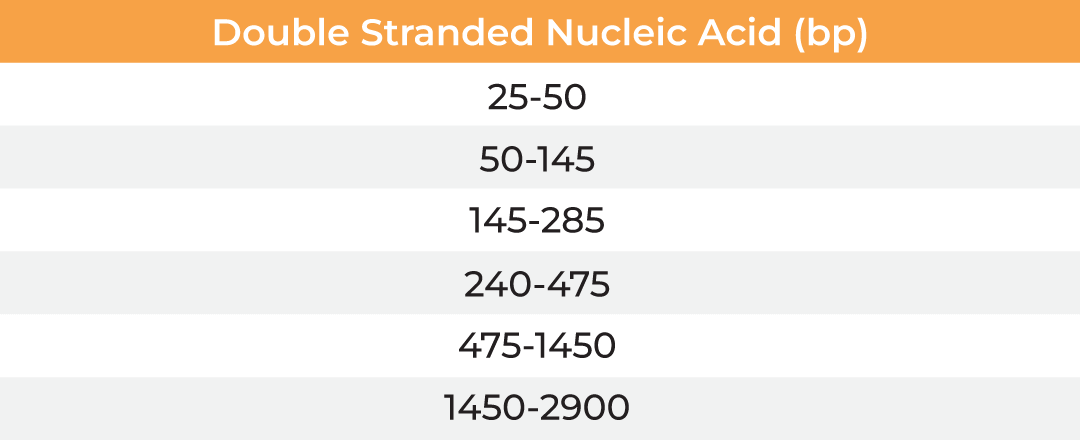
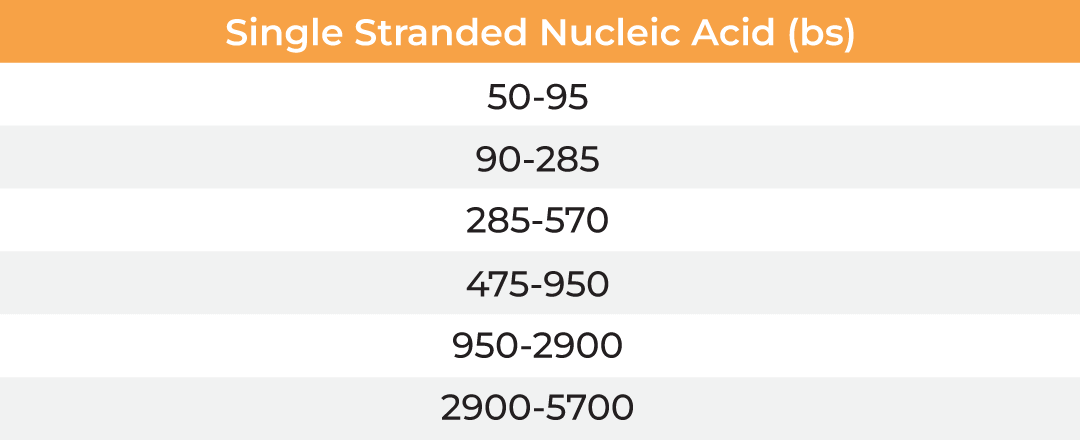
Table: Membrane MWCO selection for various biomolecules
Versatile for a Broad Range of Scientific Workflows
Efficiently process lipid nanoparticles, liposomes, and polymeric nanoparticles for drug delivery and therapeutic efficacy
Optimize the protein preparative workflow, enabling fast and gentle processes, including concentration, formulation, desalting, and refolding
Ensure gentle and efficient removal of small unconjugated molecules from a variety of crude biomolecular labeling reactions
Simplify in vitro synthesis of RNA, and linear or plasmid DNA, by efficient concentration and buffer exchange using our user-friendly system
Streamline the fast harvesting of cells, extracellular vesicles, enzymes, and Virus-Like Particles (VLPs) while ensuring high product yields and quality
Gently concentrate and exchange buffers for Adeno-associated Virus Vectors (AAVs), bacteriophages, and lentiviruses, preserving their structure for effective applications
Optimize the formulation of DNA, RNA, and polysaccharide vaccines for optimal, stable, and cost-efficient results
Webinars
Uncover the ease of use, scalability, and reusability of µPulse for lab scale formulation of recombinant L-asparaginase and other biomolecules.
Discover how the nanoparticles are processed in a fast, single step and walk-away manner with the µPulse TFF system.
Experience the single-step and scalable purification of ADCs and other macromolecules modified with small molecules using the µPulse.
Explore the ease of use, and cost-effectiveness of µPulse for processing VLPs and other macromolecules compared to dead-end units.
Application Notes
Learn about the time efficiency, cost effectiveness and gentleness of µPulse for refolding denatured proteins compared to equilibrium dialysis.
Explore the efficiency, fast processing, and gentleness of the miniaturized µPulse TFF system for concentration and buffer exchange of protein samples.
Publications
FAQs
Which filtration method is best for nucleic acids, proteins, or viral vectors?
Tangential Flow Filtration (TFF) is the optimal method for processing nucleic acids due to its gentle, scalable, and high-recovery design. TFF minimizes shear stress and maintains the integrity of sensitive samples, while its continuous flow prevents rapid fouling. For nucleic acids, TFF efficiently removes contaminants and enables buffer exchange into formulation buffers. The µPulse and aµtoPulse systems implement TFF with single-use chips, ensuring no cross-contamination and consistent, reproducible results.
TFF vs dialysis for buffer exchange in protein purification - which is better?
TFF is superior to dialysis for most buffer exchange applications in protein purification due to its speed, efficiency, and scalability. TFF systems like the µPulse or aµtoPulse complete buffer exchange in minutes to hours, whereas dialysis often requires 12–24 hours with frequent buffer changes. For most research and bioprocessing workflows requiring speed, consistency, and scalability, TFF is the recommended method.
What is the main difference between dead-end filtration (DEF) and tangential flow filtration (TFF) for lab-scale ultrafiltration?
The main difference is how fluid flows across the membrane. In dead-end filtration (DEF), the sample flows directly into the membrane, causing retained material to accumulate and rapidly clog the filter, which can lead to variable recovery and higher product loss. In contrast, tangential flow filtration (TFF) moves the sample parallel to the membrane surface, continuously sweeping away retained species to reduce fouling and maintain stable flux. This matters for lab-scale ultrafiltration because TFF provides better control of transmembrane pressure and shear, enabling higher recovery, improved reproducibility, and gentler processing of sensitive biomolecules compared to DEF.